Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Agronomía Colombiana
versão impressa ISSN 0120-9965
Agron. colomb. vol.31 no.3 Bogotá set./dez. 2013
CROP PHYSIOLOGY
Gas exchange and mass distribution of the cowpea (Vigna unguiculata [L.] Walp.) under water deficit
Intercambio gaseoso y distribución de biomasa de caupí (Vigna unguiculata [L.] Walp.) bajo déficit hídrico
Carlos Enrique Cardona-Ayala2, Alfredo Jarma-Orozco1, Hermes Araméndiz-Tatis1, Marvin Perneth-Montaño1 and César Augusto Vergara-Córdoba1
1Department of Agronomy and Rural Development, Faculty of Agricultural Sciences, Universidad de Córdoba. Monteria (Colombia). ccardonaayala@yahoo.com2Doctorate in Natural Sciences for Development, Instituto Tecnológico de Costa Rica. Cartago (Costa Rica).
Received for publication: 10 July, 2013. Accepted for publication: 1 November, 2013.
ABSTRACT
Drought tolerance is important for the survival and productivity of plants in environments where drought periods are increasing as a result of climate variability attributable to natural causes and climate change caused by human activities. The objective of this study was to evaluate the dynamics of photosynthesis (A), stomatal conductance (gs) and intrinsic water-use efficiency (WUE=A/gs) as a function of soil moisture content over a period of drought and the post-stress recovery of 14 cowpea genotypes. The studied genotypes tolerated soil moisture tensions close to -2 Mpa with no permanent wilting. Starting at a soil hydric potential of -0.7 MP a, decreases in photosynthesis (A), stomatal conductance (gs) and transpiration (E) were evident, as well as an increase in A/gs, which varied by genotype. Estimating with regression models allowed for the discrimination of the degrees of drought tolerance between the cultivars. At 4 days after resuming hydration, no significant differences were found between the means of A, gs, A/gs and E, suggesting drought tolerance in all genotypes. The genotypes: L-047 and L-034 conserved between 4 and 6 leaves, displaying the highest delayed leaf senescence during drought. Furthermore, they presented the highest biomass at 16 days post-stress recovery.
Key words: stomatal conductance, photosynthesis, drought, delayed leaf senescence.
RESUMEN
La tolerancia a sequía es importante para la supervivencia y productividad de las plantas en ambientes con aumentos de sequía, como resultado de la variabilidad climática por causas naturales y, el cambio climático por actividades humanas. El objetivo de este estudio fue evaluar las dinámicas de fotosíntesis (A), conductancia estomática (gs) y la eficiencia en el uso del agua (EUA=A/gs) en función del contenido de humedad del suelo durante un periodo de sequía, y la recuperación pos-estrés en 14 genotipos de caupí. Los genotipos estudiados toleraron tensiones de humedad del suelo cercanas a -2 Mpa, sin presentar marchitamiento permanente. A partir de potencial hídrico del suelo de -0,7 Mpa, fueron evidentes las disminuciones en A y gs, así como el aumento en A/gs, las cuales variaron con el genotipo; sus estimaciones con modelos de regresión permitieron discriminar grados de tolerancia a sequía entre cultivares. A los 4 días después de reasumir la hidratación, no se encontraron diferencias significativas entre las medias de tales parámetros. Los genotipos L-047 y L-034 conservaron entre 4 y 6 hojas, se mostraron como los de mayor retraso en senescencia foliar durante la sequía y presentaron mayor biomasa a los 16 días de recuperación post-stress.
Palabras clave: conductancia estomática, fotosíntesis, sequía, senescencia foliar tardía.
Introduction
The Cowpea (Vigna unguiculata [L.] Walp.) is one of the most important food legumes in the tropic and sub-tropic regions (Cardona-Ayala et al., 2013), where drought is a major production constraint and is always a potential problem due to low and erratic rainfall (Agbicodo et al., 2009; Ahmed and Suliman, 2010).
The physiological strategies of plants against microclimatic changes are directed toward reducing water loss; one strategy is stomatal closure, which reduces transpiration but also restricts the entry of CO2 into plants, decreasing the rate of photosynthesis and the translocation of assimilates to different organs of the plant as well as reducing the pressure gradient necessary for the entry of nutrients through the root, a situation that is aggravated with increasing temperatures and radiation (DaMatta, 2004).
In general, drought stress induces an array of morphological, physiological, biochemical, and molecular responses, in which photosynthesis is affected by a reduction in stomatal conductance (gs), which decreases the diffusion of CO2 through the stomata into the intercellular spaces and then, through the mesophyll toward carboxylation sites (Singh and Reddy, 2011). Another limitation is of a biochemical nature, known as non-stomatal limitation, and is related to the limitation of photophosphorylation, regeneration of RuBp (ribulose 1,5-bisphosphate) and Rubisco activity (Singh and Reddy, 2011).
In the cowpea, a water deficit decreases stomatal conductance, leaf water potential and productivity of grains, which appear in a wide variability in drought tolerance, according to the degree of water deficit (Nascimento, 2009). Studies conducted by Oliveira et al. (2005) show that stomatal conductance is an indicator of water stress in the cowpea and report values between 0.03 and 0.18 mol H2O m2O s-1O. The highest recorded values, when comparing genotypes under the same water stress conditions, indicate more tolerance to drought (Nascimento, 2009).
The root/aerial part ratio is also an important trait in studies of drought adaptation because water absorption can be enhanced through an extensive and deep root system to allow water absorption in a greater volume of soil (Reis and Hall, 1987). In the cowpea, long root density, rooting depth and root dry matter per unit area are parameters that characterize the root system and can be used as drought tolerance criteria along with specific leaf area (Matsui and Singh, 2003).
Furthermore, it was found that the detrimental effects of drought in the photosynthetic apparatus can last for weeks or even months, so that the ability to recover a stressed plant's photosynthetic capacity can be associated with the ability of the stomata to reopen, partially or completely, and of protein synthesis to overcome the damage to the photosynthetic apparatus (Kozlowski and Pallardy, 1996). The objective of this study was to determine the drought tolerance of 14 genotypes of cowpea obtained from a heterogeneous-homozygous population, assessing physiological responses related to gas exchange and biomass in early stages of the species cycle, for use as selection criteria of improved genotypes.
Materials and methods
Location. An experiment was conducted in a greenhouse covered with plastic between November of 2012 and February of 2013 at the Universidad de Córdoba, Colombia (8º48 'N, 78º53' W). The climatic classification of the region is tropical dry forest, with a dry period from December to April, an annual average temperature of 27.4ºC, precipitation of 1,346 mm/year, 85% relative humidity (RH) and 2,108 h sunshine per year (Palencia-Severiche et al., 2006). Experimental material. Thirteen cowpea lines obtained from a heterogeneous- homozygous population called Criollo-Córdoba (included as a control), pre-selected by yield and nutritional quality, under suitable hydration conditions, were subjected to water stress starting at 16 d after sowing. The lines were planted in 8.5 L-plastic pots, filled with disturbed alluvial soil from the Sinú Valley of a silty loam texture.
Experimental design. We used a randomized complete block design with 14 treatments and three replications. 252 pots were used, 18 per genotype; three seeds were sowed per pot and thinning was carried out at 6 d after sowing, leaving one seed per pot.
Water dynamics of the substrate. To characterize the water dynamic of the substrate and its relationship with the gas exchange parameters, a moisture retention curve was obtained at water potentials of 0, 0.01, 0.03, 0.05, 0.1, 0.5, 1.0 and 1.5 MP a along with their corresponding gravimetric moisture content, using the hydric tension container method, combined with the membrane under pressure. A water retention curve relates soil water content with suction (matric potential=water potential) and reflects the ability of a soil or substrate to retain water depending on the exerted suction (tension); its characterization is necessary to know the hydraulic properties of soils (López-Canteñs et al., 2010).
With the moisture content at 0.03 MP a (field capacity) and bulk density of the soil, the daily amount of water needed to replenish the evapotranspired plants was determined by difference in weight, in order to ensure a field capacity threshold until day 16. At that time, the water supply was suspended, when the plants had two fully expanded trifoliate leaves.
In each pot, a plastic bag was used to prevent loss of substrate by the lower holes. After 32 d of drought, the plants were rehydrated for 16 d. The total study duration was 64 d. The average air temperature was 31.67±7.92°C, the maximum mean 45.41±2.81°C, recorded between 09 and 15 h, and the minimum average 24.24±0.79°C, and recorded between 04 and 06 h. The maximum recorded temperature was 50.9°C and the minimum was 22.7°C. The average relative humidity was 69.59±23.42%, with values fluctuating between 22 and 70% during the day and between 80 and 97% at night. These measurements were taken automatically with a Humidity & Temperature Datalogger Model RH520 (Extech Instruments Corporation, Nashua, NH). Measurement of soil water content. The water content of the soil (SWC) was determined every 4 d, at depths of 6, 12 and 18 cm, using two pots with plants of the same age. Drying of the samples was done at 105°C for 24 h; simultaneously, the volumetric soil moisture was measured with a TDR 300 at a depth of 12 cm in all the pots.
Measurements of gas exchange. Measurements of gas exchange parameters: photosynthesis (A), stomatal conductance (gs), transpiration (E), as well as the intrinsic water-use efficiency (WUE=A/gs), temperature and vapor pressure deficit (VPD ) were recorded every a d during 32 d of drought and 16 d of rehydration, using the infrared gas analyzer (IRGA) model CIRAS-2 portable photosynthesis (PP Systems International, Amesbury, MA). These measurements were recorded from a leaflet of the second, third or fourth fully expanded leaf from the apex of each selected plant, between 10 and 13 h.
Direct and indirect measurements of growth and development of plants. From the 20th d after withholding irrigation, the number of trifoliate leaves without signs of wilting and the presence of reproductive organs was recorded: flowers and developing pods. After 16 d of rehydration, the dry mass of the stem and leaves (vegetative biomass), reproductive structures (reproductive biomass: flowers and pods), and total mass were measured.
Statistical analysis. The relationship between soil volumetric moisture content and soil water potential was tested for polynomial, logarithmic and exponential functions and the best fit regression was selected.
The relationships between the soil water content and the different parameters of gas exchange (A, gs, E and WUE) were tested for polynomial, linear, logarithmic and exponential functions and the best fit regressions were selected. To select models, the following diagnostic criteria suggested by Rincón (2009) were taken into account: coefficient of determination (R2), mean square error, studentized residuals (si), externally studentized residuals (ti), Cook's distance (Di), influence of each observation on its prediction (DFFITS i), and the impact of each observation on the residual sum of squares (Q(i)), for which the procedures: GLM, RE G and IML of SAS® v. 9.2 (SAS, 2008) were used.
In order to estimate the effects of the variation of soil moisture content on the direct measurements of growth and development of the genotypes, analyzes of variance were conducted for each variable. For dry biomass, the transformation square root of x+1 was used.
Results and discussion
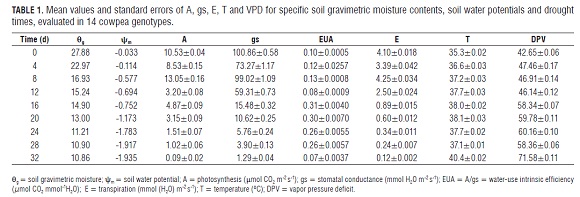
Soil moisture content and water potential According to the laboratory determinations and the estimated values using the shown equation (Fig.1) for 0.03 MP a equivalent to 0.3 atmospheres (field capacity), the substrate used in the experiment showed a moisture content of 27.88%, while at 1.5 Mpa equivalent to 15 atmospheres, corresponding to the permanent suction wilting point, where much of the plants wither, the moisture content was 12.03%.
At 32 d of drought, the soil moisture content was 10.86% and the soil water potential was estimated at -1.9 Mpa. Considering the fact that the leaf water potential and soil water potential are in balance at pre-dawn (Tardieu and Simonneau, 1998; Sellin, 1999), the cowpea is classified as moderately drought tolerant (Boyer, 1978). The latter author reported nonlethal values of -1, 4 to -2.5 MP a for this species, assuming that a lethal water potential in many plants is in the range of -1.4 to -6.0 MPa.
Gaseous exchange under water stress
The combined analysis of the 14 cowpea genotypes showed that after 12 d of suspension of the water supply, with a soil gravimetric moisture of 15.24% (45.34% of the moisture at field capacity) and a soil water potential of -0.7 MP a, A, gs and E decreased (Tab.1). The plants showed a deviation of physiology before water stress, since the average net photosynthesis decreased 55.08%, stomatal conductance 41.2% and transpiration, 39.02%, suggesting that stomatal regulation was the biggest limitation of photosynthesis (Singh and Reddy, 2011). At the same time, the intrinsic water-use efficiency (A/ gs) increased nearly 100%, from a soil water potential of -0.75 Mpa and soil gravimetric moisture content of 14.90%. Simultaneously, the leaf temperature and vapor pressure deficit (VPD ) increased 6.8% and 8.18%, respectively, due to stomatal closure (Salah and Tardieu, 1997; Tardieu and Simonneau, 1998).
The progressive decrease of A, gs and E suggests the morphological and metabolic mechanisms' activation of adaptation, to counteract the soil moisture deficit (Taiz and Zeiger, 2010; Singh and Reddy, 2011). In particular, at 16 d of drought, stomatal conductance showed an average reduction of 84.52% and continued decreasing progressively until day 32.
The reduction of gs, due to the closure of the stomata, occurs when the mesophyll begins to dehydrate and is regulated by abscisic acid (ABA) (Moreno, 2009). Although, in this study, the ABA content was not measured, the values of gs suggest an increase of the phytohormone in the leaves, due to its redistribution from the chloroplast of mesophyll cells and the synthesis and transport from the roots of this hormone (Taiz and Zeiger, 2010). ABA is released to the apoplast and reaches the guard cells through the transpiration stream (Zhang and Outlaw, 2001; Moreno 2009).
ABA causes the loss of potassium, chlorine or malate in guard cells, which causes decreases in cell volume due to cytoplasm water exit, leading to stomatal closure (Moreno, 2009; Taiz and Zeiger, 2010).
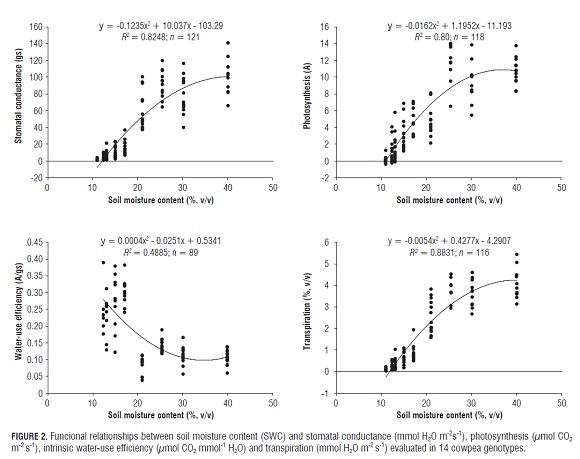
The combined analysis of 14 genotypes showed quadratic relationships between gas exchange parameters and SWC (Fig.2). Decreases in photosynthesis, stomatal conductance and transpiration varied progressively, with soil drying as a result of evapotranspiration. After 24 d of water supply suspension, with a gravimetric moisture content of 11.21% and a soil water potential of –1.8 MP a, the average of these parameters were significantly reduced and, after 32 d of drought, net assimilation and stomatal conductance were in the proximity of zero, a situation that presaged the collapse of the plants (Agbicodo et al., 2009). However, the 14 genotypes maintained green stems and only some leaves showed signs of senescence and basal regrowth in some plants; also, there was a reduction in the size of the stems and leaves as a result of decreased turgor pressure (Taiz and Zeiger, 2010). Similar results were reported by Hall (2004) for the cowpea, with a water potential of -1.8 MPa.
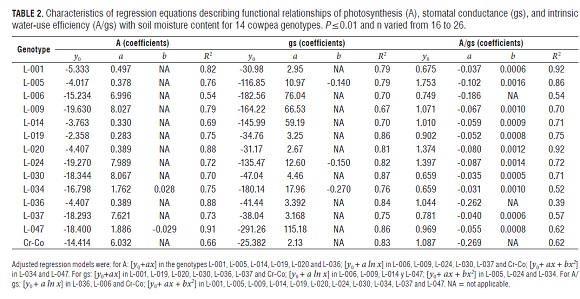
The magnitudes of A and gs decreased at different rates among the genotypes, with effects that were linear, logarithmic or quadratic during the drought period (Tab.2), suggesting genetic differences in drought tolerance among the genotypes. Likewise, the increase of A/gs was expressed at different rates between the studied genotypes.
Gradual decreases of A and gs indicate partial or complete closure of stomata to reduce water loss through transpiration. It has been reported that stomatal closure as a result of decreasing soil moisture content, mediated by changes in the water status of the root (Bates and Hall, 1981), is a response to a water deficit in the cowpea (Cruz de Carvalho et al., 1998; Anyia and Herzog, 2004; Hamidou et al., 2007; Singh and Reddy, 2011) and, according to Nascimento (2009), higher stomatal conductance values indicate a high drought tolerance.
In this sense, intrinsic water-use efficiency (A/gs) has been recognized as a measure of carbon gain per unit of water loss and is inversely proportional to the ratio of intercellular and environmental CO2 (Ci/Ca) concentrations (Singh and Reddy, 2011). It is evident that a higher intrinsic water-use efficiency and a higher photosynthetic rate can improve crop yield under water stress (Condon et al., 2002; Parry et al., 2005; Singh and Reddy, 2011).
An efficient mechanism to transfer information from the roots to the leaves may partially explain the drought avoidance shown by this plant (Bates and Hall, 1981; Moreno, 2009). Furthermore, the osmotic adjustment due to accumulation of inorganic and organic solutes and other strategies associated with adaptation to constraining environmental factors could be occurring (Rodríguez, 2006; Moreno, 2009; González-Rodríguez et al., 2011).
The accumulation of free proline in Helianthus annus under water stress has been reported, but its synthesis takes place earlier or later, depending on the degree of drought tolerance of the cultivar (Unyayar et al., 2004). In cowpea plants under water stress, significant increases of proline favoring osmotic adjustment have been reported (Lobato et al., 2008). Also, increases of total carbohydrates and amino acids and decreased amounts of total protein have been reported (Costa et al., 2008; Lobato et al., 2009).
The best fit functional relationships were linear, quadratic and logarithmic for A and gs, while, for A/gs, they were quadratic and logarithmic, in relation to the decrease in soil volumetric water content (Tab.2). Singh and Reddy (2011) fitted linear equations for A and exponential ones for gs and A/gs in 15 genotypes originating from the USA, Senegal, Nigeria and India. The trend is the same: photosynthesis and the stomatal conductance decrease, whereas intrinsic water-use efficiency increases when the soil moisture content decreases.
The variation in rates of A, gs and A/gs in each genotype allows for the estimation of these parameters at a time interval as a function of soil moisture content (Singh and Reddy, 2011) and, in this study, allowed for the discrimination of the degrees of potential drought tolerance among the cultivars.
Gaseous exchanges after rehydration
At 4 d after resuming hydration, the analysis of variance showed no significant difference among the means of A, gs, A/gs and E. The mean values observed were 15.5±3.6 µmol (CO2) m2O s-1O, 104.6±19.9 mmol (H2O) m2O s-1O and 0.15±0.02 µmol (CO2) mmol-1 (H2O), respectively. These values are similar to and, in some cases, higher than those obtained when starting the water supply suspension, suggesting drought tolerance in all genotypes. Similar results were found at 8, 12 and 16 d after resuming hydration (data not shown).
When plants that are subjected to a water deficit period are irrigated, the rate of photosynthesis may or may not return to previous levels, depending on the genetic material, severity and duration of the drought and relative humidity (Kozlowski and Pallardy, 1996). Plants with the ability to photosynthesize as they did before a water stress are more drought-tolerant. In this regard, Cruz de Carvalho et al. (1998) showed that stomatal conductance and the net assimilation rate measured during and after a water stress treatment are reliable parameters to identify drought tolerance in Phaseolus vulgaris and Vigna unguiculata.
Moreover, it was found that the detrimental effects of drought on the photosynthetic apparatus can last for weeks or even months, so the ability to recover a stressed plant's photosynthetic capacity can be associated with the ability of stomata to reopen, partially or completely, and of protein synthesis to overcome the damage to the photosynthetic apparatus (Kozlowski and Pallardy, 1996).
Electron transport seems to be very resistant to inhibition under a water deficit, while the photosynthetic metabolism and phosphorylation appear to be more susceptible to dehydration. Phosphorylation might be sensitive to the toxic effects of high concentrations of magnesium accompanying the chloroplast water removal of dehydrated leaves (Kozlowski and Pallardy, 1996; Taiz and Zeiger, 2010).
Number of leaves and dry mass of organs
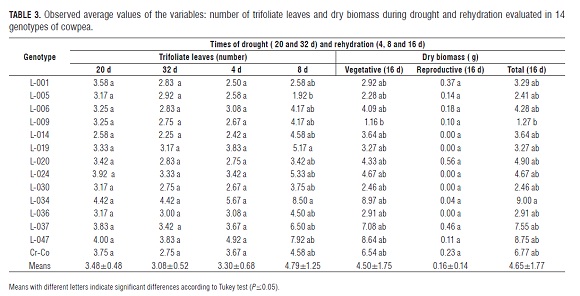
At 32 d after sowing (16 d of drought), some genotypes developed flowers and initiated the formation of 1 or 2 underdeveloped pods. At 48 d (32 d of drought), the values of A, gs and E were in the vicinity of zero and, from this state, the water supply was resumed to observe post-stress recovery for 16 d. Reproductive biomass, only present in some genotypes, was represented by flowers and underdeveloped pods (Tab.3).
No significant differences were found in the number of trifoliate leaves between 20 and 32 d (Tab.3), the time in which the water was retained in the soil matrix with suctions between -1 and -2 MP a. At 32 d of progressive drought, 8 genotypes showed 2 to 3 leaves, 4 between 3 and 4, and only 2, L-047 and L-034, conserved between 4 and 6 leaves, displaying the highest delayed leaf senescence. Delayed leaf senescence has been reported as a characteristic that contributes to drought adaptation (Muchero,2009) because it increases the survival of the plants after a dry period and it permits the emission of a second stream of pods once the water supply is restored (Ehlers and Hall, 1997 Hall, 2004; Agbicodo et al., 2009).
On the other hand, 8 d after resuming hydration, the analysis of variance showed significant differences between the genotypes and Turkey's test allowed for the separation of L-034 from L-005. However, the former did not differ significantly from the rest of the materials tested. Moreover, significant differences were found in total biomass (represented in the stems, leaves and reproductive structures), indicating that the genotype L-034 accumulated more biomass than L-009. However, L-034 was not statistically different from the 12 remaining genotypes. The differences in the partition of assimilates were reflected in the total dry biomass, explained in part, by the differences in the parameters of gas exchanges.
It is noteworthy that 50% of cultivars did not develop flowers or that those that did, did so at a very slow rate; while for the remaining cultivars, the reproductive biomass represented 3 to 18%. This suggests that, in the genotypes studied, the mechanisms of adaptation to drought: escape, avoidance and tolerance (Mitra, 2001), although they could operate jointly or separately, are expressed differently. However, the ways in which these responses are associated with these mechanisms are not yet known (Agbicodo et al., 2009).
Additionally, the early flowering genotypes possess delayed leaf senescence and undetermined growth habits and should exhibit drought adaptation and stability in yield in various environments (Ehlers and Hall, 1997). It is possible that these characteristics are associated with a higher density of deeply distributed roots, which allow for a higher water absorption (Matsui and Sing, 2003; Polanía et al., 2012) and might suggest drought avoidance. With estimates of stomatal conductance, photosynthesis, intrinsic water-use efficiency and total dry biomass, the genotypes: L-034, L-047, L-006 and L-001 were identified as the most potentially drought tolerant because they presented higher values of A, gs and A/gs between 12 and 28 d of drought, which corresponded to a soil water content between 15.24 and 10.90%, equivalent to soil water potentials of between -0.7 and -1.9 MP a and higher values of total dry biomass.
Conclusions
Rates of photosynthesis and stomatal conductance decreased starting at a soil water potential of -0.70 MP a, while intrinsic water-use efficiency increased progressively with soil drying. Photosynthesis and stomatal conductance reached zero values when the soil moisture content was 10.86% and the soil water potential was close to -2 Mpa without producing permanent wilting of plants in any genotype. Decreases in photosynthesis and stomatal conductance and increased intrinsic water-use efficiency under water stress conditions varied with the genotypes and the estimates, with regression equations, allowed for discrimination of the degrees of drought tolerance among the cultivars. Fourteen genotypes showed full recovery of the gas exchange parameters after resuming the water supply.
Acknowledgements
The authors thank the División de Investigación of the Universidad de Córdoba for its financial support of this research. Special thanks to the staff of the laboratories of Plant Breeding, Plant Physiology and Soils of the Agronomic Engineering program.
Literature cited
Agbicodo, E.M., C.A. Fatokun, S. Muranaka, R.G.F. Visser, and C.G. Linden Van Der. 2009. Breeding drought tolerant cowpea: constraints, accomplishments, and future prospects. Euphytica 167, 353-370. [ Links ]
Anyia, A.O. and H. Herzog. 2004. Genotypic variability in drought performance and recovery in cowpea under controlled environment. J. Agron. Crop Sci. 190(2), 151-159. [ Links ]
Bates, L.M. and A.E. Hall. 1981. Stomatal closure with soil water depletion not associated with changes in bulk leaf water status. Oecologia 50, 62-65. [ Links ]
Boyer, J.S. 1978. Water deficit and photosynthesis. pp. 154-191. In: Kozlowski, T.T. (ed.). Water deficits and plant growth. Academic Press, London. [ Links ]
Cardona-Ayala, C., A. Jarma-Orozco, and H. Araméndiz-Tatis. 2013. Mecanismos de adaptación a sequía en caupí (Vigna unguiculata (L.) Walp.). Una revisión. Rev. Colomb. Cienc. Hortic. 7(2), 277-288. [ Links ]
Condon, A.G., R.A. Richards, G.J. Rebetzke, and G.D. Farquhar. 2002. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 42, 122-131. [ Links ]
Costa, R.C.L., A.K.S. Lobato, C.F. Oliveira-Neto, P.S.P. Maia, G.A.R. Alves, and Laughinghouse. 2008. Biochemical and physiological responses in two Vigna unguiculata (L.) Walp. cultivars under water stress. J. Agron. 7, 98-101. [ Links ]
Cruz de Carvalho, M.H., D. Laffray, and P. Louguet. 1998. Comparison of the physiological responses of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Environ. Exp. Bot. 40, 197-207. [ Links ]
DaMatta, F.M. 2004. Exploring drought tolerance in coffee: a physiological approach with some insights for plant breeding. Braz. J. Plant Physiol. 16(1), 1-6. [ Links ]
Ehlers, J. and A. Hall. 1997. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res. 53, 187-204. [ Links ]
González-Rodríguez, H., I. Cantú-Silva, R.G. Ramírez-Lozano, M.V. Gómez-Meza, M. Pando-Moreno, and J.M. López-Hernández. 2011. Potencial hídrico xilemático en cuatro especies arbustivas nativas del noreste de México. Rev. Chapingo 17, 97-109. [ Links ] Hall, A. 2004. Breeding for adaptation to drought and heat in cowpea. Europ. J. Agron. 21, 447-454. [ Links ]
Hamidou, F., G. Zombre, O. Diouf, N. Diop, S. Guinko, and S. Braconnier. 2007. Physiological, biochemical and agromorphological responses of five cowpea genotypes (Vigna unguiculata (L.) Walp.) to water deficit under glasshouse conditions. Biotechnol. Agron. Soc. Environ. 11(3), 225-234. [ Links ]
Kozlowski, T.T. and S.G. Pallardy. 1996. Physiology of woody plants. 2nd ed. Academic Press, San Diego, CA. [ Links ]
Lobato, A.K.S., C.F. Oliveira-Neto, R.C.L. Costa, B.G. Santos-Filho, FJ.R. Cruz, and H.D. Laughinghouse. 2008. Biochemical and physiological behavior of Vigna unguiculata (L.) Walp. under water stress during the vegetative phase. Asian J. Plant Sci. 7, 44-49. [ Links ]
Lobato, A.K.S, R.C.L. Costa, C.F.O. Neto, B.G.S. Filho, G.A.R. Alves, J.M.N. Freitas, F.J.R. Cruz, C.A. Marochio, and G.K. Coimbra. 2009. Responses of the pigments and carbon metabolism in Vigna unguiculata cultivars submitted to water deficit. Res. J. Biol. Sci. 4, 593-598. [ Links ]
López-Canteñs, G., J. Herrera-Puebla, A. Ostos-Santos, L. Lizarraga- Mendiola, and J. Hernández-Ávila. 2010. Aplicación de modelos matemáticos para la obtención de curvas de retención de humedad del suelo. Rev. Latin. Rec. Nat. 6(1), 44-50. [ Links ]
Matsui, T. and B.B. Singh. 2003. Root characteristics in cowpea related to drought tolerance at the seedling stage. Exp. Agr. 29 (1), 29-38. [ Links ]
Mitra, J. 2001. Genetics and genetic improvement of drought resistance of crop plants. Current Sci. 80, 758-763. [ Links ]
Moreno, F. 2009. Respuesta de las plantas al estrés por déficit hídrico. Una revisión. Agron. Colomb. 27(2), 179-191. [ Links ]
Muchero, W., J.D. Ehlers, T.J. Close, and P.A. Roberts. 2009. Mapping QTL for drought stress-induced premature senescence and maturity in cowpea (Vigna unguiculata (L.) Walp.). Theor. Appl. Genet. 118, 849-863. [ Links ]
Nascimento, S.P.D. 2009. Efeito do deficit hídrico em feijão-caupi para entificação genótipos com tolerância à seca. M.Sc. thesis. Agricultural Science Center, Universidad Federal do Piauí, Teresina, Brazil. [ Links ]
Oliveira, A.D., E.J. Fernandes, and T.J.D. Rodrigues. 2005. Condutância estomática como indicador de estresse hídrico em Feijão. Eng. Agríc. 25(1), 86-95. [ Links ]
Palencia-Severiche, G., T. Mercado-Fernández, and E. Combatt-Caballero. 2006. Estudio agroclimático del departamento de Córdoba, Universidad de Córdoba, Monteria, Colombia. [ Links ]
Parry, M.A.J., J. Flexas, and H. Medrano. 2005. Prospects for crop production under drought: research priorities and future directions. Ann. Appl. Biol. 147, 211-226. [ Links ]
Polanía, J.A., I.M. Rao, S. Mejía, S.E. Beebe, and C. Cajiao. 2012. Características morfo-fisiológicas del frijol común (Phaseolus vulgaris L.) relacionadas con la adaptación a sequía. Acta Agron. 61(3), 197-206. [ Links ]
Reis, G.G. and A.E. Hall. 1987. Relações hídricas e atividade do sistema radicular em Eucalyptus camaldulensis Dehn. em condições de campo. Rev. Árvore 11(1), 43-55. [ Links ]
Rincón, L. 2009. Curso básico de modelos lineales. Departamento de Publicaciones, Universidad Santo Tomás, Bogota. [ Links ]
Rodríguez, L. 2006. Implicaciones fisiológicas de la osmorregulación en plantas. Agron. Colomb. 24(1), 28-37. [ Links ]
Salah, B.H. and F. Tardieu.1997. Control of leaf expansion rate of droughted maize plants under fluctuating evaporative. Plant Physiol. 893-900. [ Links ]
SAS. 2008. User's guide SAS/ST AT® version 9.2. SAS Institute, Cary, NC . [ Links ]
Sellin, A. 1999. Does Pre-Dawn water potential reflect conditions of equilibrium in plants and soil water status? Oecologica 20(1), 51-59. [ Links ]
Singh, S.K. and K.R. Reddy. 2011. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photochem. Photobiol. B: Biol. 105, 40-50. [ Links ]
Taiz, L. and E. Zeiger. 2010. Plant physiology. 5th ed. Sinauer Associates, Redwood, CA. [ Links ]
Tardieu, F. and T. Simonneau. 1998. Variability of species among stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviours. J. Exp. Bot. 49, 419-432. [ Links ]
Unyayar, S., Y. Keles, and E. Unal. 2004. Proline and ABA levels in two sunflower genotypes subject to water stress. Bulg. J. Plant Physiol. 30 (3-4), 34-47. [ Links ]
Zhang, S.Q. and W.H. Outlaw. 2001. Abscisic acid introduced into the transpiration stream accumulates in the guard cell apoplasto and causes stomatal closure. Plant Cell Environ. 24, 1045-1054. [ Links ]