Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.32 no.1 Bogotá Jan./Apr. 2014
https://doi.org/10.15446/agron.colomb.v32n1.40544
http://dx.doi.org/10.15446/agron.colomb.v32n1.40544
PLANT BREEDING, GENETIC RESOURCES AND MOLECULAR BIOLOGY
1 National Phytosanitary Laboratory Diagnosis, Tibaitatá Research Center, Instituto Colombiano Agropecuario (ICA). Mosquera (Colombia). jorge.angel@ica.gov.co; eeebraitr@unal.edu.co
Received for publication: 29 October, 2013. Accepted for publication: 19 March, 2014.
ABSTRACT
Four DNA citrus plant tissue extraction protocols and three methods of DNA extraction from vector psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae) were compared as part of the validation process and standardization for detection of huanglongbing (HLB). The comparison was done using several criterias such as integrity, purity and concentration. The best quality parameters presented in terms of extraction of DNA from plant midribs tissue of citrus, were cited by Murray and Thompson (1980) and Rodríguez et al. (2010), while for the DNA extraction from psyllid vectors of HLB, the best extraction method was suggested by Manjunath et al.(2008).
Key words: Candidatus Liberibacter, Diaphorina citri, Psyllidae, vectors.
RESUMEN
En el proceso de validación y estandarización para la detección de huanglongbing (HLB) se compararon protocolos de extracción de ADN, cuatro a partir de tejido vegetal de cítricos y tres a partir del psílido vector Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Para la comparación de los protocolos se utilizaron los criterios de integridad, pureza y concentración. Los protocolos citados por Murray and Thompson (1980) y Rodríguez et al. (2010) fueron los que presentaron los mejores parámetros de calidad en términos de extracción de ADN a partir de la nervadura central del tejido vegetal de cítricos. En la extracción de ADN a partir del psílido vector de HLB, el método que mejores resultados arrojó fue el reportado por Manjunath et al. (2008).
Palabras clave: Candidatus Liberibacter, Diaphorina citri, Psyllidae, vectores.
Introduction
The disease known as citrus huanglongbing (HLB) was first detected in Asian countries and Africa and more recently in American countries such as Argentina (SENASA, 2013), Costa Rica (SFE, 2011), Belize (Manjunath et al., 2010), Cuba (Martínez et al., 2009), Mexico (NAPPO, 2009), Dominican Republic (Matos et al., 2009), USA (Halberth, 2005; Manjunath et al., 2008) and Brazil (Teixeira et al., 2005), which implied important losses to the citrus industry (Bové, 2006). HLB is caused by alpha-proteobacteria non-cultivated genus Candidatus Liberibacter inhabiting the phloem of citrus plants (Da Graca, 1991; Tsai and Liu, 2000; Tsai et al., 2002) and is spread by vegetative propagation and insect vectors, reason why an effective control is difficult (Hung et al., 2004; Manjunath et al., 2008).
According to the etiological agent, its genome and the influence of temperature on the expression of symptoms in the host plant, HLB has been divided into Asian, African and American variants (Halbert and Manjunath, 2004). The Asian and American variants are transmitted by the psyllid vector Diaphorina citri and are worldwide known as Candidatus Liberibacter asiaticus (Garnier et al., 2000) and Candidatus Liberibacter americanus (Teixeira et al., 2005), while the African variant Candidatus Liberibacter africanus is transmitted by the psyllid Trioza erytreae and Diaphorina citri (Bové, 2006; Lin et al., 2010).
In 2007, the psyllid vector Diaphorina citri was first reported in Colombia by the Colombian Agricultural Institute (ICA) in citrus crops and seedlings in the departments of Valle del Cauca and Tolima; later the insect was found in the departments of Risaralda, Caldas, Quindio, Antioquia, Cordoba, Cesar, Bolivar, Atlantico, Norte de Santander, Santander, Casanare, Meta, Huila, Cauca, Nariño and Cundinamarca, infesting 95% of the citrus area in the Central Pacific, Orinoco and Atlantic regions of Colombia (Ebratt et al., 2011), due to Colombian's proximity to countries where the presence of the insect vector and disease has been detected, puts the country at high risk for this serious disease of citrus.
Conventionally, visual symptoms indicate the presence of HLB (Roistacher, 1991), but over time more reliable detection systems have been developed based on electronic microscopy, specific fluorescent marker substances to HLB (Schwarz, 1968) and enzyme linked immunosorbent assays with monoclonal antibodies (ELISA) (Gao et al., 1993). Developed detection methods using PCR and real time PCR, were based on the 16S ribosomal DNA region analysis and several other regions of the bacterium,s genome (Hocquellet et al., 1999; Lin et al., 2010; Morgan et al., 2012).
In order to standardize and apply molecular methods for detection of HLB in citrus regions around the country and to establish a rapid and reliable method for diagnosis, four DNA extraction protocols were compared from citrus leaf tissue, and three DNA extraction methods from Diaphorina citri psyllids. The basic parameters evaluated in each method were: concentration, purity, efficiency and integrity.
Methods and materials
Plant material
Ten samples of lemon mandarin (Citrus aurantifolia) leaf tissue from trees between 10 and 15 years old were collected in the area of the Instituto Colombiano Agropecuario of Mosquera (ICA-Tibaitata), and were then transported in styrofoam coolers with the appropriate cooling conditions until their final storage location. The next day, the leaves were washed with sterilized water and dried with paper towels, to begin the DNA extraction process.
Capture of Diaphorina citri psyllids
Adult individuals were collected using entomological nets, while the nymphs of D. citri were directly taken from symptomatic trees in different phenological stages (vegetative, flowering or harvest) such as lemons (Citrus limon), oranges (Citrus sinensis) and mandarins (Citrus reticulata) between 4 and 20 years old, located in the municipalities of Sasaima and La Mesa, in the department of Cundinamarca. Afterwards, they were placed in 1.5 mL Eppendorf tubes with alcohol (96% concentration) and taken to the National Laboratory of Phytosanitary Diagnosis LNDF-ICA-Tibaitata, where they were stored at room temperature until the DNA extraction was performed.
DNA extraction from plant tissue of citrus
Four DNA extraction methods previously reported were compared. Three of them were based on CTAB and 2-mercaptoethanol (Thompson et al., 1983; Murray and Thompson, 1980; Rodríguez et al., 2010) and one reported by Qiagen (a commercial kit). Ten samples were tested by each method. The whole DNA was extracted from the midrib of each leave of the collected samples, which were finely cut and pulverized using liquid nitrogen according to the four protocols. All were compared through four quality parameters: concentration, purity, efficiency and integrity, which depend on detection of the disease during the analytical procedures based on PCR (García-Cañas et al., 2004), and the pollutants coming from the extraction process that can inhibit these reactions (Hughes and Moody, 2007).
Extraction of DNA from D. citri psyllids
To perform the extraction of DNA from psyllids three methods were compared: Manjunath et al. (2008), Aljanabi et al. (1998) and Teixeira et al. (2005). In all the cases, the sample was made up by six nymphs or six adults. The concentration, purity and integrity of the three quality indicators were evaluated.
Determination of the integrity, concentration, and purity of DNA extracts
Extracted DNA was run on an agarose gel 1.2% to determine its integrity (undegraded DNA, and without DNA presence scanning) according to a scale from one to five (one meaning completely degraded DNA and five non-degraded DNA). The concentration of all samples was determined by a spectrophotometric analysis using a NanoDrop ND-1000 (Thermo Fisher Scientific, Wilmington, DE), according to the manufacturer's instructions and procedure. Its purity was assessed by a 260/280 nm ratio, with and approximate value of 1.8. This value indicates the DNA is free of contaminants. The effectiveness of the DNA extraction process was calculated by the following equation:
where, A is the DNA performance (ng of ADN/mg of plant tissue); B, is the volumen of the DNA extract (mL of H20 or buffer in which the extracted DNA pellet was resuspended), in accordance to the used extraction method); C, is the DNA concentration in the volume of extract (ng mL-1); D, is the plant tissue weight used in each DNA extraction method (mg).
Statistical analysis
The statistical analysis was performed using the "t-Student" test, specifically the couple comparison products of SAS (Statistical Analysis System) software version 8.0 (SAS Institute, 2002), which integrated both plant and insect samples, previous verification of the assumptions of normality and homoscedasticity.
Discussion and results
Extraction effectiveness and integrity of the DNA absorbance range 260/280 nm, from leaf tissue
In each of the methods for extracting DNA from leaf tissue different factors were evaluated: concentration (ng mL-1), performance (ng mg-1 DNA from plant tissues), the purity by the absorbance ratio 260/280 nm, indicating the presence or absence of protein contaminants, and the integrity of the relation to the non-degradation of the total DNA. Once the parameters were evaluated, it was observed that the DNA obtained from the Murray and Thompson (1980) protocol, showed the required parameters, and its concentration and purity determined via equipment Nanodrop 1000 (Figs. 1 and 2) was adequate. Thompson,s protocol (1983), was a good indicator which enabled establishing that although its concentration was optimized for the needs of amplification (396.8 ng DNA/mg plant tissue, on average), its purity (1.2) indicated the presence of polyphenols due probably to the absence of PVP for DNA extraction, and its integrity (2.4) was also deficient, as degradation was evidenced, which can decrease the efficiency of the PCR (Holden et al., 2003).
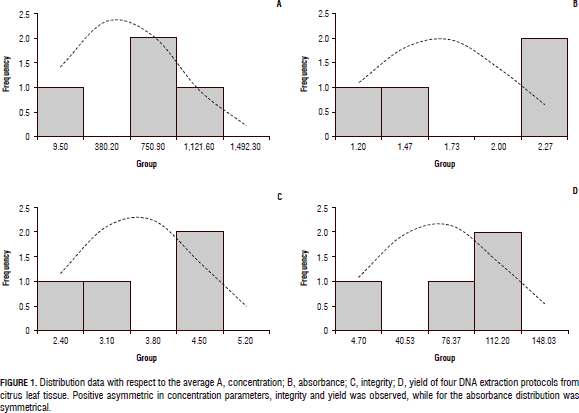
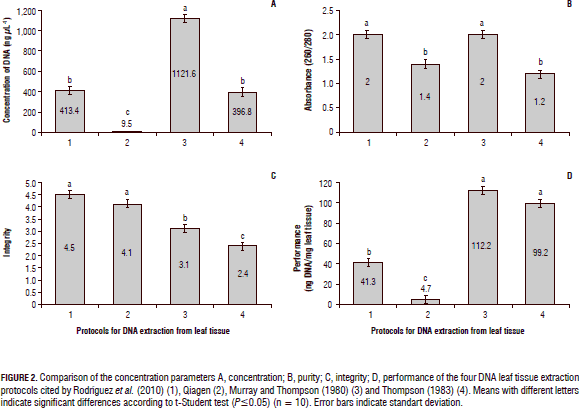
The protocol reported by Rodríguez et al. (2010), starting from 500 mg of plant tissue, had optimal quality parameters with a high purity of the DNA (1.77 to 2.06), and an appropriate integrity (4.5) defined by non degraded DNA, the DNA concentrations extracted were relatively high compared with the other extraction methods evaluated (Fig. 2). Figure 3 shows the integrity of DNA extractions obtained by this protocol, which was the best of the four methods analyzed. Finally, it was demonstrated that when assessing the commercial Qiagen method, yield was inferior to the other procedures tested in this study. Additionally, the absorbance rate indicated the presence of contaminants such as not hydrolyzed proteins and polyphenols, which could not be completely removed during the process. However, the integrity of the method had an acceptable value compared to the other protocols (4.1).
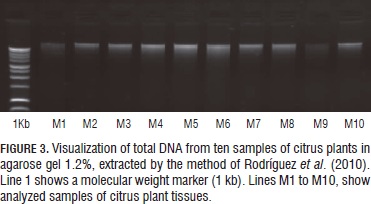
Concentration and integrity of the DNA extractions, and the absorbance range 260/280 nm, from psyllids
The visualization of the results for each of the evaluated parameters (Figs. 4 and 5) to compare DNA extraction methods from psyllid D. citri, reported by Manjunath et al. (2008), Aljanabi et al. (1998) and suggested by Teixeira et al. (2005), shows that the latter did not provide good quality parameters, such as the low concentration and poor integrity of the DNA. This is similar to observations reported by Aljanabi et al. (1998), where although the concentration of nucleic acids was high (230.6 ng mL-1), the quality of DNA was degraded (1.1) and its absorbance (0.9) indicated the presence of contaminants in the samples.
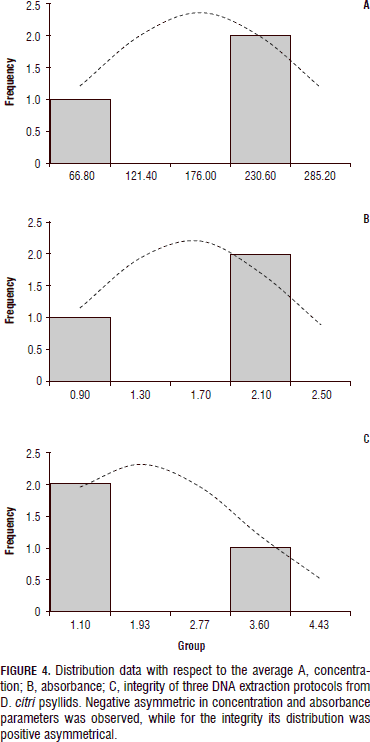
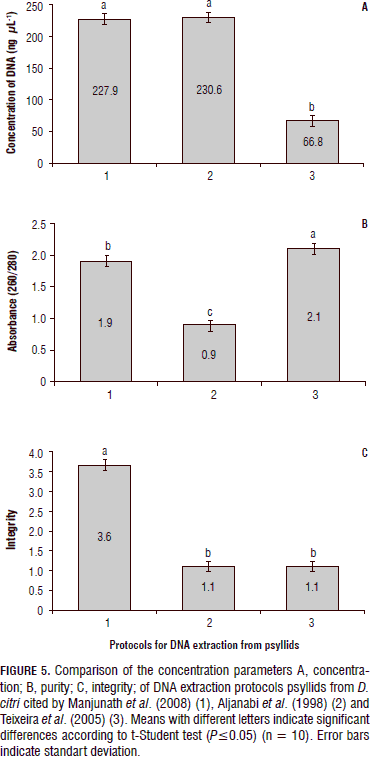
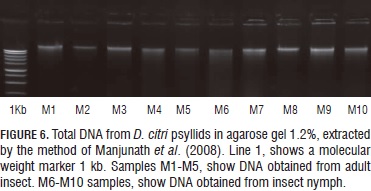
Finally, the protocol reported by Manjunath et al. (2008) allowed establishing the samples proven with this method had the best integrity among all the other tested protocols (Fig. 6), and outstanding concentration of nucleic acids and a better absorbance ratio that indicated that the samples were free of contamination.
Because the concentration of the bacteria,s variants that causes HLB can be very low in the insect vector and especially in citrus leaf tissue, causing that the symptoms of this disease are not always easily observed, consequently it is very important to have a method that give the best indicators of purity, quantity and quality, so although certain protocols in this study showed good results in one or more of the parameters evaluated, should not be used as a starting point for the diagnosis of HLB, because contaminants such as polyphenols and no hydrolyzed proteins could lead to inhibition of the PCR reactions and lead to false negatives. According this, the statistical analysis conducted by the testing method "t-Student ", significant differences were found between the methodologies evaluated for both DNA extractions made from leaf tissue and those made from D. citri. The DNA extraction method for psyllids reported by Manjunath et al. (2008), was the best quality indicators obtained and presented significant difference (P≤0.0001) compared to the methods reported by Aljanabi et al. (1998) and Teixeira et al. (2005). Regarding the protocols used from plant tissue, the difference between the methods which presented the best quality parameters (Murray and Thompson, 1980; Rodríguez et al., 2010), with the protocols of Qiagen and the reported by Thompson (1983), was highly significant (P≤0.0001).
Conclusions
The protocols with best results as quality indicators to perform DNA extractions from leaf tissue of citrus plants were cited by Murray and Thompson (1980) and Rodríguez et al. (2010). Also, the method by which the best results were obtained for this procedure from psyllids, was the proposed by Manjunath et al. (2008).
Acknowledgements
The authors express their gratitude to Keremane Manjunath, researcher at the American Department of Agriculture (USDA) and Chandrika Ramadugu, researcher with the Department of Botany and Plant Sciences at the University of Riverside (CA), for their technical support and for providing positive control DNA citrus plants infected with bacteria Ca. L. americanus, Ca. L. asiaticus and Ca. L. africanus. Additionally, to Diva do Carmo Teixeira, researcher of the Fund for Citrus Plant Protection (Fundecitrus) of Brazil, who kindly provided the positive DNA citrus control plants from that country, infected with bacteria Ca. L. americanus and Ca. L. asiaticus. Finnally to Colciencias, for funding this research.
Literature cited
Aljanabi, S.M., M.S. Loiacono, R.T. Lourenco, M. Borges, and M. Tigano. 1998. RAPD analysis revealing polymorphism in egg parasitoids of soybean stink bugs (Hemiptera: Pentatomidae). An. Soc. Entomol. Bras. 27(3), 413-420. [ Links ]
Bové, J.M. 2006. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88(1), 7-37. [ Links ]
Da Graca, J.V. 1991. Citrus greening disease. Annu. Rev. Phytopathol. 29, 109-136. [ Links ]
Ebratt-Ravelo, E.E., L.T. Rubio-González, V.A. Costa, Á.P. Castro-Ávila, E.M. Zambrano-Gómez, and J.E. Ángel-Díaz. 2011. Diaphorina citri (Kuwayama, 1907) and Tamarixia radiata (Waterson, 1922) in citrus crops of Cundinamarca, Colombia. Agron. Colomb. 29(3), 487-493. [ Links ]
Gao, S.M., M. Garnier, and J. Bove. 1993. Production of monoclonal antibodies recognizing most Asian strains of the greening BLO by in vitro immunization with an antigenic protein purified from the BLO. pp. 244-249. In: Moreno, P., J.V. da Graca, and L.W. Timmer (eds.). Proc. 12th Conference of the International Organization of Citrus Virologists. University of California, Riverside, CA. [ Links ]
García-Cañas, V., A. Cifuentes, and R. González. 2004. Detection of genetically modified organisms in foods by DNA amplification techniques. Crit. Rev. Food Sci. 44, 425-436. [ Links ]
Garnier, M., S. Jagoueix-Eveillard, P.R. Cronje, H.F. Le Roux, and J.M. Bove. 2000. Genomic characterization of a liberibacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal of 'Candidatus Liberibacter africanus subsp. Capensis'. Int. J. Syst. Evol. Microbiol. 50, 2119-2125. [ Links ]
Halbert, S.E. and K.L. Manjunath. 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Fla. Entomol. 87(3), 330-353. [ Links ]
Halbert, S.E. 2005. The discovery of huanglongbing in Florida. p. 50. In: Proc. 2nd International Citrus Canker and Huanglongbing Research Workshop. Vol. H-3. Florida Citrus Mutual. Orlando, FL. [ Links ]
Hocquellet, A., P. Toorawa, J.M. Bove, and M. Garnier. 1999. Detection and identification of the two Candidatus Liberobacter species associated with citrus huanglongbing by PCR amplification of ribosomal protein genes of the beta operon. Mol. Cell. Probes 13, 373-379. [ Links ]
Holden, M.J., J.R. Blasic, L. Bussjaeger, C. Kao, L.A. Shokere, D.C. Kendall, L. Freese, and G.R. Jenkins. 2003. Evaluation of extraction methodologies for corn kernel (Zea mays) DNA for detection of trace amounts of biotechnology-derived DNA. J. Agric. Food Chem. 51, 2468-2474. [ Links ]
Hughes, S. and A. Moody. 2007. PCR: methods express. Scion Publishing, Bloxham, UK. [ Links ]
Hung, T.-H., S.-C. Hung, C.-N. Chen, M.H. Hsu, and H.J.P. Su. 2004. Detection by PCR of Candidatus Liberibacter asiaticus, the bacterium causing citrus huanglongbing in vector psyllids: application to the study of vectorâpathogen relationships. Plant Pathol. 53, 96-102. [ Links ]
Lin, H., C. Chen, H. Doddapaneni, Y. Duan, E.L. Civerolo, X. Bai, and X. Zhao. 2010. A new diagnostic system for ultra-sensitive and specific detection and quantification of Candidatus Liberibacter asiaticus, the bacterium associated with citrus huanglongbing. J. Microbiol. Meth. 81, 17-25. [ Links ]
Manjunath, K.L., S.E. Halbert, C. Ramadugu, S. Webb, and R.F. Lee. 2008. Detection of 'Candidatus Liberibacter asiaticus' in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathol. 98, 387-396. [ Links ]
Manjunath, K.L., C. Ramadugu, V.M. Majil, S. Williams, M. Irey, and R.F. Lee. 2010. First report of the citrus huanglongbing associated bacterium 'Candidatus Liberibacter asiaticus' from sweet orange, Mexican lime, and Asian citrus psyllid in Belize. Plant Dis. 94(6), 781. [ Links ]
Martínez, Y., R. Llauger, L. Batista, M. Luis, A. Iglesia, C. Collazo, I. Peña, J.C. Casín, J. Cueto, and L.M. Tablada. 2009. First report of 'Candidatus Liberibacter asiaticus' associated with huanglongbing in Cuba. Plant Pathol. 58, 389. [ Links ]
Matos, L., M.E. Hilf, and J. Camejo. 2009. First report of 'Candidatus Liberibacter asiaticus' associated with citrus huanglongbing in the Dominican Republic. Plant Dis. 93, 668. [ Links ]
Morgan, J.K., L. Zhou, W. Li, R.G. Shatters, K. Manjunath, and D. Yong-Ping. 2012. Improved real-time PCR detection of 'Candidatus Liberibacter asiaticus' from citrus and psyllid hosts by targeting the intragenic tandem-repeats of its prophage genes. Mol. Cell. Probes 26, 90-98. [ Links ]
Murray, M.G. and W.F. Thompson. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8(19), 4321-4326. [ Links ]
NAPPO, North American Plant Protection Organization. 2009. Detection of huanglongbing 'Candidatus Liberibacter asiaticus' in the municipality of Tizimin, Yucatan, Mexico. In: Phytosanitary alert system. Official pest reports, www.pestalert.org/oprDetail.cfm?oprID=384; consulted: February, 2014. [ Links ]
Rodríguez Q., C.G., E.I. Alanís M., J. Velázquez M., and I.H. Almeyda L. 2010. Optimización de la técnica de extracción del DNA de plantas de cítricos para el diagnóstico del HLB (on-line). In: Memoria 1er Simposio Nacional sobre Investigación en el Manejo del Psílido Asiático de los Cítricos y el Huanglongbing en México. Monterrey, Mexico. [ Links ]
Roistacher, C.N. 1991. Techniques for biological detection of specific citrus graft transmissible diseases. pp. 12-158. In: Roistacher, C.N. (ed.). Handbook for detection and diagnosis of graft-transmissible diseases of citrus. FAO, Rome. [ Links ]
Schwarz, R.E. 1968. Indexing of greening and exocortis through fluorescent marker substances. pp. 118-124. In: Price, W.C. (ed.). Proc. 4th Conf. Intl. Organ. Citrus Virol. University of Florida, Gainesville, CA. [ Links ]
SENASA, Servicio Nacional de Sanidad y Calidad Agroalimentaria. 2013. La amenaza del HLB (Huanlongbing de los cítricos) para la citricultura nacional. In: www.senasa.gov.ar/contenido.php?to=n&in=11&io=18493; consulta: February, 2014. [ Links ]
SFE, Servicio Fitosanitario del Estado. 2011. Autoridades del SFE confirman presencia de "Dragón Amarillo" en árboles de la Zona Norte. In: www.sfe.go.cr/documentos/comunicados/2011/21_02_2011_SFE_confirma_presencia_HLB_en_zona_norte.pdf; consulted: February, 2014. [ Links ]
Teixeira, D.C., J.L. Danet, S. Eveillard, E.C. Martins, W.C.J. Junior, P.T. Yamamoto, S.A. Lopes, R.B. Bassanezi, A.J. Ayres, C. Saillard, and J.M. Bove. 2005. Citrus huanglongbing in Sao Paulo State, Brazil: PCR detection of the 'Candidatus' Liberibacter species associated with the disease. Mol. Cell. Probes 19, 173-179. [ Links ]
Thompson, R.D., D. Bartels, N.P. Harberd, and R.V. Flavell. 1983. Characterization of the multigene family coding for HMW glutein subunits in wheat using cDNA clones. Theor. Appl. Genet. 67, 87-96. [ Links ]
Tsai, J.H. and Y.H. Liu. 2000. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J. Econ. Entomol. 93, 1721-1725. [ Links ]
Tsai, J.H., J.J. Wang, and Y.H. Liu. 2002. Seasonal abundance of the Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) in southern Florida. Fla. Entomol. 87, 446-451. [ Links ]













