Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.32 no.1 Bogotá Jan./Apr. 2014
https://doi.org/10.15446/agron.colomb.v32n1.41362
http://dx.doi.org/10.15446/agron.colomb.v32n1.41362
CROP PROTECTION
1 Faculty of Basic and Applied Sciences, Universidad Militar Nueva Granada. Cajica (Colombia). juan.filgueira@unimilitar.edu.co
Received for publication: 27 December, 2013. Accepted for publication: 19 March, 2014.
ABSTRACT
The rose downy mildew disease, caused by Peronospora sparsa Berkeley, is one of the most important that affect rose crops in Colombia. To manage this disease, flower growers must deal with high-costs due to the excessive application of fungicides, but without good results. Studies on P. sparsa behavior have shown its narrow relationship with environmental conditions. In this study, the temperature effect was evaluated during the infection and sporulation of P. sparsa in Charlotte leaflets, a susceptible commercial variety, through an environmental controlled conditions system. Infection and sporulation were observed at different temperatures in a range of from 4 to 40°C. Infection with the absence of or very low sporulation was observed at 4°C. The most favorable pathogen responses were between 15 and 18°C in terms of inoculum concentration and sporulation percentage. There was no infection or leaflet change above 35°C. According to the results, sporulation can occur from 4 to 33°C, confirming the fact that P. sparsa is able to reproduce throughout a wide temperature range.
Key words: ornamental plants, Peronosporales, phytopathogen, pseudofungi, sporulation, infection.
RESUMEN
La enfermedad del mildeo velloso, causada por Peronospora sparsa Berkeley, es una de las más importantes que afectan los cultivos de rosa en Colombia. Para manejar esta enfermedad, los floricultores se deben enfrentar a los altos costos producidos por las reiteradas aplicaciones de fungicidas, sin buenos resultados. Estudios basados en el comportamiento de P. sparsa, han demostrado su estrecha relación con las condiciones ambientales. En este trabajo se evaluó el efecto de la temperatura en la infección y esporulación de P. sparsa, en foliolos de rosa, de la variedad comercial Charlotte, en un sistema de condiciones ambientales controladas. La infección y esporulación fue observada a diferentes temperaturas, en un rango de 4 a 40°C. A 4°C fue evidente la infección y la esporulación que se presentó fue baja y poco frecuente. Las respuestas más evidentes en cuanto a concentración de inóculo y porcentaje de esporulación fueron a 15 y 18°C. A temperaturas por encima de 35°C, no hubo infección o cambios en los foliolos. De acuerdo con los resultados, puede haber esporulación abundante a temperaturas entre 4 y 33°C, confirmando que P. sparsa tiene la habilidad de reproducirse en un amplio rango de temperaturas.
Palabras clave: plantas ornamentales, Peronosporales, fitopatógeno, pseudohongo, esporulación, infección.
Introduction
For about 40 years, floriculture has been important in Colombia because it contributes greatly to increases in the foreign exchange and job creation (Quirós, 2001). According to the AsociaciónColombiana de Exportadores de Flores (Asocolflores), by 2012, flower production had become mainly for export and Colombia is the second largest producer country of cut flowers in the world after the Netherlands, contributing 95% of the total supply of flowers (Quirós, 2001; Asocoloflores, 2013). Nevertheless, roses have the highest market value of cut flowers with the United States being the principal importer (Merino, 2004; Yong, 2004; Asocolflores, 2013).
Rose crops are affected by a diversity of pathogens, which cause losses in plant production, quality and productivity (Castillo et al., 2010). Among them, Peronospora sparsa Berkeley is responsible for the downy mildew disease; this disease has threatened and limited commercial rose crops on the Bogota Plateau and producers have been facing high-costs due to the intensive use of fungicides to manage it (Castillo et al., 2010).
Common symptoms that P. sparsa produces in leaflets are mainly: spots that range from purple to brown over the upper surface, while in the lower surface there is the grayish or white mycelium that corresponds to the sporangiophores and sporangia that produce the downy appearance. The leaflets may be curled and, finally, severe defoliation occurs (Gómez and Arbeláez, 2005a; Ayala et al., 2008; Gómez and Filgueira, 2012). The sporulation can also occur on the stems, calyx and buds. In buds, the infection leads to death or mummification, which enables the development of secondary diseases caused by opportunistic pathogens such as Botrytis sp. (Gómez and Arbeláez, 2005a; Restrepo, 2006; Ayala et al., 2008).
Some observations have shown that there is a narrow relationship between environmental conditions and the appearance of downy mildew (Gómez and Filgueira, 2012). Variables such as relative humidity, light intensity, photoperiod and free water on the leaves, favor the occurrence of this disease (Hildebrand and Sutton, 1984a). In Nordic countries, information on the biology of P. sparsa is limited because greenhouses are conditioned and the disease is not present (De Vis, 1999). In Colombia, rose downy mildew research has been advancing (Gómez and Arbeláez, 2005a, 2005b; Gómez and Filgueira, 2012) but information related to temperature is still scarce; however, it is known that its effect is significant on pathogen development (Giraldo et al., 2002; Gómez and Arbeláez, 2005b).
Consequently, it is useful to develop systems to control and reproduce different environmental conditions that promote the occurrence of this pathogen in order to know its behavior. Therefore, the objective of this research was to further the biology of P. sparsa, evaluating the temperature effect on infection and sporulation in Charlotte leaflets, a susceptible commercial variety.
Materials and methods
Environmental controlled conditions system
The environmental controlled conditions system (ECCS) was built at the Plant Biotechnology Laboratory at the Military "Nueva Granada" University, in Cajica, Colombia.
To isolate the ECCS from the changing external temperature, the room had a hermetic door and walls covered with Styrofoam and Duralfoil® and there was also a 100 cm wide x 150 cm long x 100 cm high acrylic cube where the temperature assessments took place.
Obtaining and maintaining P. sparsa inoculum in moist chamber
Uninfected rose leaflets were collected from the greenhouse and taken to the laboratory. They were disinfected and put in petri dishes to inoculate and create a humidity chamber environment, as described by Soto and Filgueira (2009). Four leaflets were placed in each petri dish, up to a total of 40 for each temperature assessment.
After leaflet inoculation, the petri dishes were placed under room conditions (18°C, 75% relative humidity and 12/12 h photoperiod) for 48 h. Afterwards, the inoculum drops on the leaflet surface were dried and the Petri dishes were transferred to the ECCS for 72 h for disease development, a period in which data were recorded.
Determination of the temperature effect on P. sparsa development
In the ECCS, it was possible to manage, control and maintain the chosen temperature. The warming system permitted raising the temperature up to 40°C. In the lower-temperature chamber, an air-conditioning system was employed to maintain the room temperature at 18°C, while a cooling system permitted lowering the temperature down to 4°C in the acrylic case. The cooling system consisted of a cooler whose resistor was submerged in a container with liquid antifreeze. Inside the acrylic chamber, there was a pump submerged and connected to one end of a copper hose. This hose was contained inside the acrylic chamber so the cold liquid antifreeze could lower and maintain the temperature.
The relative humidity was maintained between 90 and 99%, light intensity was 790 lx with a photoperiod of 12/12 h, maintained as previously described by Soto and Filgueira (2009). These variables were optimal during the assessments according to the results reported by Monroy and Filgueira (2009) and Soto and Filgueira (2009).
To measure the temperature and relative humidity inside the acrylic chamber, there was an Oakton® rotor hygrothermograph (Oakton Instruments, Vernon Hills, Il), which recorded these variables for a period of 7 d. In addition, there were a Fisher® thermohygrometer (Thermo Fisher Scientific, Waltham, MA) and a Silber Brand® mercury thermometer (Berlin, Germany) for permanent hygrothermograph calibration.
The evaluated temperatures inside the ECCS were divided into: low; 4 and 9°C, medium; 15 and 18°C and high; 30, 33, 35 and 40°C. Each temperature assessment had tree repetitions. After 7 d of assessments, the temperature effect was evaluated according to the disease development in the rose leaflets, as previously described by Soto and Filgueira (2009).
Macroscopic changes on the leaflets were annotated and the data for sporulation (total infected leaflets/total inoculated leaflets*100) and the amount of sporangia (sporangia/mL) produced during the experiment were recorded. The sporangia were quantified with a hemocytometer and observed in a light microscope at 100x. Additionally, the sporangia germination was tested to know the viability, as a quality parameter of the pathogen sporulation. The sporangia were observed in a light microscope at 470x. This procedure was previously described by Gómez and Filgueira (2012).
Statistical analysis
The statistical analysis of the data obtained from the sporulation percentage, inoculum concentration and sporangia germination was carried out using software R, free version 2.13.2. ANOVA, Shapiro-Wilk and Tukey test were used.
Results and discussion
Macroscopic observations of rose leaflet infected with P. sparsa
The leaflet observations for the 3 d after inoculation showed the common disease symptoms, which included blackish or brown spots on the upper surface of the leaflets and, on the lower surface, the sporangiophores and sporangia, producing the downy and grayish appearance (Fig. 1).
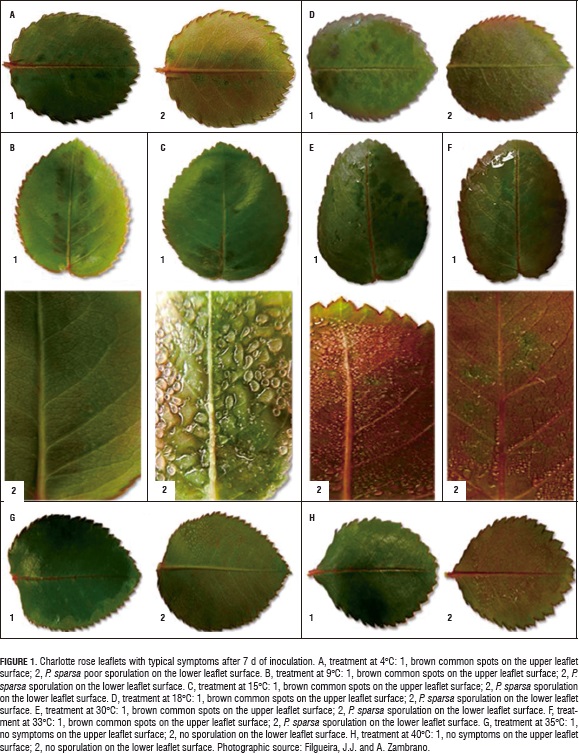
There was no sporulation at 4°C (Fig. 1A). According to the results presented by Aegerter et al. (2003), sporulation does not occur at 4°C; however, in the present study, at this temperature, common spots were observed, indicating the invasion and colonization of pathogen tissue. The P. sparsa sporulation begun to be evident at 9°C (Fig. 1B), but, at the medium temperatures: 15 and 18°C, the larger sporulation percentages were evident (60.4 and 48.5%, respectively). In that range, the sporangiophore growth was from the central vein to the margin of the leaflet where P. sparsa grew; the leaflet in this site was green or brown, while, where sporulation had not occurred yet, it was reddish, a characteristic mature color. On the upper surface, the black spots were evident (Fig. 1C and 1D). At high temperatures, P. sparsa sporulated in the range of 30°C in all of the three repetitions (Fig. 1E and 1F). At 33°C, for two repetitions, the common symptoms on the upper surface occurred but sporulation did not, as was observed at 4°C. There was no sporulation or color change in the leaflets from 35°C and up (Fig. 1G and 1H), indicating that this pathogen is not able to survive under these conditions.
In the cases where the symptoms where observed on the upper surfaces of the rose leaflets but without sporulation, it meant that the pathogen was latent while the environmental conditions were favorable, so sporulation was possible. The same observations were reported by Hildebrand and Sutton ( 1984a) for Peronospora destructor and Giraldo et al. (2002), Aegerter et al. (2003), and Gómez and Arbeláez (2005b) for P. sparsa, in which the assessment temperatures affected the latent sporulation period. This suggests, contrary to what is commonly thought in the flower industry, that greenhouse warming in the early morning is an option that allows for the management of this disease, thus avoiding the overuse of fungicides that, as we know, are not effective for downy mildew control.
During the evaluation period, the room conditions were favorable, so P. sparsa could infect and invade the rose leaflets, although the sporulation capacity was affected when the pathogen was exposed to high temperatures. This corresponds to reports by Achar (1998) and Williams et al. (2007) from their studies on Peronospora parasitica and Plasmopara viticola, respectively; indicating that in their evaluations, there was no sporulation at 35°C, or even infection.
Sporulation percentage and sporangia amount produced under environmental controlled conditions
In the environmental controlled conditions room it was possible to determine the temperature effect on P. sparsa biology. This variable affected the relationship between the amount of sporulated leaflets and the amount of sporangia produced. This suggests that the range of activity of this pathogen is broad enough to develop in healthy plants and that the environmental conditions, especially the temperature, have an effect on the way the disease cycle of the pathogen is completed, which is reflected primarily in sporulation, just as authors, such as Hildebrand and Sutton (1984b) and Aegerter et al. (2003), have suggested.
The results corresponding to sporulation percentages and inoculum concentration are presented in Fig. 2. The largest sporulation percentage was at 18°C with 60.4%, then at 15°C with 48.5% and at 9°C with 40.8%, while the largest inoculum concentration was at 18 and 15°C with 12.1x104 sporangia/mL.
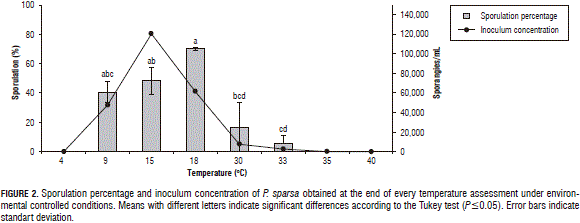
According to the statistical analysis, the sporulation percentage showed a significant difference at 18°C (P=0.00017), in comparison with the other treatments (4, 9, 30, and 33°C). The inoculum concentration, meanwhile, indicated that, between 15 and 18°C, there were no significant differences (P=0.004) but was representative of the other evaluated temperatures.
This indicates that temperature has an effect on disease development; as the temperature increases from low to medium, the amount of infected leaflets is higher, as is the amount of sporangia produced, both of which decreased when the temperature was from medium to high in value.
These observations are similar to those reported in several studies by Hildebrand and Sutton (1984a, 1984b) for the onion downy mildew caused by Peronospora destructor and by Aegerter et al. (2003) in San Joaquin valley, California, as well as Giraldo et al. (2002) and Gómez and Arbeláez (2005b) for rose downy mildew.
Although reports for P. sparsa indicate that, at temperatures above 25°C, sporulation does not occur, as Aegerter et al. (2003) reported in their study, in this research, sporulation was obtained at 30 and 33°C, albeit with the lower percentages and amounts of sporangia produced in relation to the other temperatures. This is very important since, according to the literature, in Colombia, there has not been a report of rose downy mildew sporulation in this range of temperatures. These results show that the pathogen has been adapting to the environmental conditions of the Bogota Plateau, which means that the range of activity has been expanding to the point that it sporulates at temperatures between 30 and 33°C. Recent studies have found that the abiotic historical factors that the pathogen has faced may influence the way it responds to more or less extreme conditions. This has been reported for other species of mildews, such as Pseudoperonospora cubensis (Lebeda and Cohen, 2011).
There are also reports of other species of Peronospora that are related to weather conditions and that may sporulate at temperatures from 5 to 30°C, which is the case for P. parasitica (Achar, 1998), P. destructor (Buloviené and Survielené, 2006), P. viticola (Williams et al., 2007) and P. cubensis (Lebeda and Cohen, 2011), while the optimal sporulation ranges may vary between 15 to 25, as presented in this research.
Sporangia germination of P. sparsa
The sporangia germination assessments of P. sparsa were done at the Plant Biotechnology Laboratory (Nueva Granada University Campus). The temperature was between 17 and 19°C, with an average relative humidity of 85%.
The light microscopy observations revealed the sporangia morphology at all the temperatures and showed an oval form with the entire cellular content and with long germinal tubes (Fig. 3).
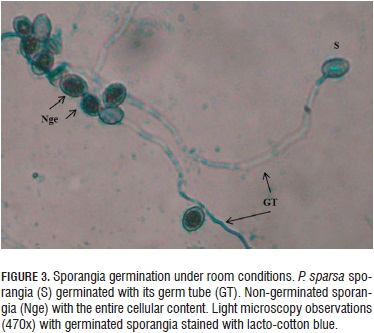
The germination assessment results showed that the geminated sporangia are viable and are able to perform new infective processes under normal weather conditions and may also form long germ tubes for invading plant parts far from where the infection process began (Gómez and Filgueira, 2012).
The sporangia germination percentages of P. sparsa were above 60% for the temperatures of 9, 15 and 18°C (Fig. 4). Gómez and Filgueira (2012) indicated that, in the first 6 to 8 h after the germination assessment, the sporangia had germinated at 70.5 to 80% under room conditions.
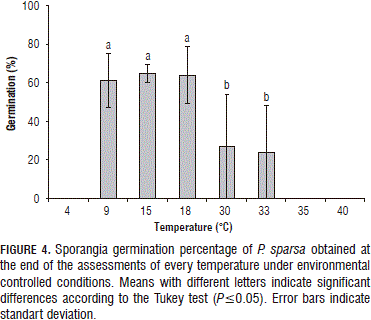
The sporangia germination did not exceed 30% in the range of 30 to 33°C (Fig. 4); however, sporangia may infect a plant even at this minimum percentage. Hildebrand and Sutton (1984a) maintained that P. destructor spores germinated after a period of 2 h at 10, 14 and 18°C and, also, that the germination capacity decreases with increasing temperatures.
The sporangia germination was slightly higher at 15°C than at 18°C, which is consistent with the study conducted by Giraldo et al. (2002), in which they found that sporangia reach germination potential after 8 h of inoculation and germinate between 5 and 15°C, albeit lower in comparison with temperatures between 15 to 20°C.
The germination tests revealed a relationship between the amount of sporangia produced and the amount of sporangia germinated since their viability was affected by the temperature at which the assessment was performed. That is to say that, at 9, 15 and 18°C, the sporangia had an increased ability to begin new infective processes in relation to higher temperatures, such as 30 and 33°C.
Conclusions
According to the results, it was observed that temperature influences the amount of sporulated leaflets and production of sporangia. The medium temperatures, between 15 and 18°C, are the most favorable for producing high infections and sporulation in different green parts of plants on the Bogota Plateau. There was very poor sporulation and low-quantity infection at 4°C; while at 30 and 33°C, sporulation was evident. The sporangia viability assessments showed that they have the ability to initiate new infective processes at these extreme temperatures (4 and 33°C) and, also, that the pathogen displays adaptation to the Bogota Plateau weather and greenhouse conditions..
The obtained results will contribute to knowledge of P. sparsa biology as well as help the flower industry search for new solutions in the management and effective control of this disease, keeping in mind that weather variables influence the pathogen infective process.
Acknowledgements
The authors wish to thank the Universidad Militar "Nueva Granada" for funding project CIAS-551.
Literature cited
Achar, P. 1998. Effects of temperature on germination of Peronospora parasitica conidia and infection of Brassica oleracea. J. Phytopathol. 146, 137-141. [ Links ]
Aegerter, B., J. Nuñez, and R. Davis. 2003. Environmental factors affecting rose downy mildew and development of a forecasting model for nursery production system. Plant Dis. 87(6), 732-738. [ Links ]
Asocolflores. 2012. Estadísticas. Rev. Asocolflores 79, 6-13. [ Links ]
Asocolflores. 2013. Boletín económico mayo de 2013. Bogota. [ Links ]
Ayala, M., L. Argel, S. Jaramillo, and M. Montoya. 2008. Diversidad genética de Peronospora sparsa (Peronosporaceae) en cultivos de rosa de Colombia. Acta Biol. Colomb. 13, 79-94. [ Links ]
Buloviené, V. and E. Survielené. 2006. Effect of environmental conditions and inoculum concentration on sporulation of Peronsopora destructor. Agron. Res. 4, 147-150. [ Links ]
Castillo, C., E. Álvarez, E. Gómez, G. Llano, and J. Zapata. 2010. Mejoramiento nutricional de la rosa para el manejo de Peronospora sparsa Berkeley, causante del mildeo velloso. Rev. Acad. Colomb. Cienc. Exact. Fis. Nat. 34(131), 137-142. [ Links ]
De Vis, R. 1999. Manejo del mildeo velloso en rosa con control climático. Acopaflor 3(6), 61-68. [ Links ]
Giraldo, S., C. García, and F. Restrepo. 2002. Influencia de la luz y la temperatura en la germinación de esporangios y en la esporulación de Peronospora sparsa Berkeley, en rosa cultivar Charlotte. Agron. Colomb. 20(3), 31-37. [ Links ]
Gómez, S. and G. Arbeláez. 2005a. Caracterización de la respuesta de tres variedades de rosa a la infección de Peronospora sparsa Berkeley, bajo condiciones de invernadero. Agron. Colomb. 23(2), 239-245. [ Links ]
Gómez, S. and G. Arbeláez. 2005b. Efecto de la temperatura en el periodo de latencia y producción de esporangios de Peronospora sparsa Berkeley en tres variedades de rosa. Agron. Colomb. 23(2), 246-255. [ Links ]
Gómez, S. and J.J. Filgueira. 2012. Monitoring the infective process of the downy mildew causal agent within micropropagated rose plants. Agron. Colomb. 30(2), 214-221. [ Links ]
Hildebrand, P. and J. Sutton. 1984a. Relationships of temperature, moisture, and inoculum density to the infection cycle of Peronospora destructor. Can. J. Plant Pathol. 6, 127-134. [ Links ]
Hildebrand, P. and J. Sutton. 1984b. Interactive effects of the dark period, humidity period, temperature and light on sporulation of Peronospora destructor. Amer. Phytopatol. Soc. 74, 1444-1449. [ Links ]
Lebeda, A. and Y. Cohen. 2011. Cucurbit downy mildew (Pseudoperonospora cubensis) biology, ecology, epidemiology, host-pathogen interaction and control. Eur. J. Plant Pathol. 129, 157-192. [ Links ]
Merino, M. 2004. Un mundo en flor. Rev. Horticultura 174, 42-48. [ Links ]
Monroy, I. and J.J. Filgueira. 2009. La humedad relativa en la infección y esporulación del mildeo velloso de la rosa Peronospora sparsa Berkeley como método para controlar la enfermedad. Rev. Asocolflores 73, 45-50. [ Links ]
Quirós, M. 2001. La floricultura en Colombia en el marco de la globalización: aproximaciones hacia un análisis micro y macroeconómico. Rev. Universidad EAFIT 122, 59-68. [ Links ]
Restrepo, F. 2006. Manejo del mildeo velloso (Peronospora sparsa) en rosas. Offset Gráfico Editores, Bogota. [ Links ]
Soto, J. and J.J. Filgueira. 2009. Efecto del fotoperiodo y la intensidad lumínica sobre la esporulación de Peronospora sparsa Berkeley, bajo condiciones controladas. Agron. Colomb. 27(2), 245-251. [ Links ]
Williams, M., P. Magarey, and K. Sivasithamparam. 2007. Effect of temperature and light intensity on early infection behavior on a Western Australian isolate of Plasmopara viticola, the downy mildew pathogen of grapevine. Austral. Plant Pathol. 36, 325-331. [ Links ]
Yong, A. 2004. El cultivo del rosal y su propagación. Cultivos Tropicales 25, 53-67. [ Links ]













