Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Agronomía Colombiana
versión impresa ISSN 0120-9965
Agron. colomb. vol.32 no.1 Bogotá ene./abr. 2014
https://doi.org/10.15446/agron.colomb.v32n1.38673
http://dx.doi.org/10.15446/agron.colomb.v32n1.38673
1 Plant Genetic Resources, TibaitatáResearch Center, Corporación Colombiana de Investigación Agropecuaria (Corpoica). Mosquera (Colombia).
2 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogota (Colombia). lerodriguezmo@unal.edu.co
Received for publication: 5 July, 2013. Accepted for publication: 19 March, 2014.
ABSTRACT
The present study was carried out in a Typic Hapludand soil in the municipality of 'El Rosal' (Colombia) and aimed to compare the yield performance of the cultivar Criolla Colombia under foliar applications of Zn chelate (0, 1, 2 and 3 kg ha-1) and edaphic applications of granulated Zn sulfated (0, 1, 2 and 3 kg ha-1). A split-plot, randomized complete block design, with four repetitions per treatment, was employed. In each category, the evaluated variables were: tuber weight and number of tubers. The results revealed that the 3.0 kg ha-1 edaphic application rendered a quadratic yield behavior with a relative increase of 7.9 t ha-1 (136%) for the first category tuber weight and 9.5 t ha-1 (68%) for total weight. In turn, the foliar application of the same dose resulted in a 5.8 t ha-1 (93%) relative increase and a first category tuber total weight increase of 3.8 t ha-1 (24%). Under the edaphic and foliar Zn applications, the number of tubers underwent 77 and 86% increases, respectively, with respect to the control. These results show the importance of Zn in photoassimilate accumulation efficiency, structure differentiation and tuber quality in this short-cycle crop.
Key words: fertilization, diploid potato, micronutrients, tubers, Andean crops.
RESUMEN
Esta investigación se realizó en el municipio de El Rosal (Colombia), en un suelo Typic Hapludand y tuvo como objetivo evaluar el efecto de la aplicación edáfica de sulfato de Zn granulado (0, 1, 2 y 3 kg ha-1), comparado con la aplicación foliar de quelato de EDTA Zn (0, 1, 2 y 3 kg ha-1) sobre el potencial del rendimiento del cultivar Criolla Colombia. Se utilizó un diseño de parcelas divididas en bloques completos al azar y cuatro repeticiones por tratamiento. Las variables evaluadas fueron: peso y número de tubérculos por categorías. Los resultados muestran que ante la aplicación edáfica de una dosis de Zn de 3,0 kg ha-1, el rendimiento presenta un comportamiento cuadrático, con un incremento relativo de 7,9 t ha-1 (136%) para el peso de los tubérculos de categoría primera, y de 9,5 t ha-1 (68%) para el peso total. Ante la aplicación foliar de la misma dosis, se observó un incremento relativo de 5,8 t ha-1 (93%) para el peso de los tubérculos de categoría primera y de 3,8 t ha-1 (24%) para el peso total. El número de tubérculos registró un aumento de 77% y de un 86% en la aplicación edáfica y foliar, con respecto al testigo, estos resultados muestran la importancia que tiene el Zn en la eficiencia de acumulación de fotosíntatos, diferenciación de sus estructuras y calidad de los tubérculos en un cultivo de ciclo corto como Criolla Colombia.
Palabras clave: fertilización, papa diploide, micronutrientes, tubérculos, cultivos andinos.
Introduction
In Colombia, the name "yellow diploid potato" (Papa Criolla) refers to those morphotypes that exhibit yellow rind and flesh (egg yolk phenotype) (Rodríguez et al., 2009). This type of potato was initially classified as Solanun phureja (Hawkes, 1990), later on as the Solanun tuberosum Phureja Group (Huamán and Spooner, 2002), and, recently, as the Solanun tuberosum Andigenum Group (Spooner et al., 2007, Rodríguez et al., 2010). Although it can be cultivated between 2,000 and 3,000 m a.s.l., its optimum range is from 2,300 to 2,800 m a.s.l. (Becerra-Sanabria et al., 2007).
Colombia is known as the number one diploid potato producer worldwide, planting 8,500 ha a year, from which a hundred thousand tons are harvested in the departments of Cundinamarca, Nariño and Boyaca (Herrera and Rodríguez, 2012), producing exports of 1,000 t yearly (Fedepapa, 2012).
Those who have managed to export this product report promising experiences due to its good international acceptance, resulting from its unique taste. However, it is necessary to solve problems related to a homogeneous, constant and sufficient supply, in order to adequately satisfy the demands of international contracts. For this reason, it is necessary to improve the production, transformation and commercialization processes (Herrera and Rodriguez, 2012), Stressing the call for crop technology research and dissemination and for the adoption of good agricultural practices (GAP) with an emphasis on the proper management of soil fertility and water.
The variety Criolla Colombia exhibits an erect growth habit, intense lilac flowers, and good development of its light green foliage. Having no dormant period and a tuber potential yield of 15 t ha-1, this plant produces rounded tubers with half-depth eyes and an intense yellow rind and flesh (Rodríguez et al., 2009).
Within potato cropping, fertilization is one of the most remarkable production costs, reaching figures of about 39% (Porras, 2005). Although Colombia has developed a strong tradition for the modernization of this crop, which has good industrialization and exportation potential (Martínezet al., 2006), supply price increments and inadequate crop management limit productivity and threaten the competitiveness of the system (Rodríguez et al., 2009). For this reason, current research aims to identify yield limiting factors and innovative cropping practices. Among the latter, those dealing with soil fertility strive for an integral and balanced nutritional management with special emphasis on micro-nutrients, an aspect that has been insufficiently investigated. Integral fertilization is one of the most efficient practices for assuring the full expression of a plant's genetic potential, resulting in better yields, both in terms of quality and quantity (Castro and Gómez, 2010).
Interest in micro-nutrients has recently captured the attention of plant nutrition and physiology specialists, since, in many agro-ecosystems, these minerals limit productivity, although this is frequently not so evident (Kirkby and Römheld, 2007). Hence, their adequate supply does not only determine considerable yield increments, but also an advantageous utilization of nitrogen and phosphorus fertilization (Kirkby and Römheld, 2007).
Micro-nutrients in potato crop fertilization (the case of Zn)
The fact that micro-nutrients are present in much lower concentrations than macro-nutrients in plant tissues indicates that they are likely to play different roles in growth and metabolism (Kirkby and Römheld, 2007), which is the most frequent case. These low concentrations reflect the role played by these nutrients as enzymatic reaction activators and as part of the prosthetic groups of metalloproteins, which are capable of catalyzing redox processes through electron transference (mainly the transition elements: Fe, Mn, Cu and Mo). Micro-nutrients are also likely to form complexes, linking an enzyme to a substrate, which is the case with Fe and Zn. Some of these minerals, such as Mn, Zn and Cu, are known to be present in superoxido diminutasa (SD) isozymes, which act as sweeping systems that eliminate toxic oxygen radicals, thus protecting biomembranes, DNA, chlorophyll and proteins (Kirkby and Römheld, 2007). The main functions of micro-nutrients are presented in Tab. 1.
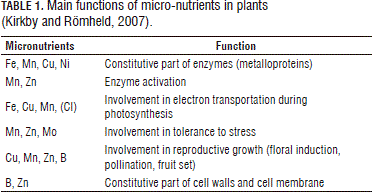
Zinc is absolutely essential to healthy plant growth and optimum yields in all agricultural and horticultural crops (Alloway, 2004). In contrast with Fe, Mn, Cu and Mo, Zn is a transition element that is, therefore, not subjected to valence changes. Zn is absorbed as a divalent cation (Zn+2) and transported through the xylem either freely or as a part of organic acids. Not oxidized or reduced by plant metabolism, Zn+2 acts as an enzymatic cofactor or metallic component (Marschner, 1995). Some of these metalloenzymes bind to other enzymes and their substrates, while, in other cases, Zn forms tetrahedral complexes with N and O, which are particularly coupled to S in a variety of organic compounds (Vallee and Auld, 1990; Kochian, 1991; Kirkby and Römheld, 2007).
Higher plants produce few Zn containing enzymes, which play catalytic, coactivating and structural roles (Vallee and Auld, 1990; Vallee and Falchuk, 1993). Among these enzymes, we can count carbonic anhydrase (CA), RNA polymerase and alcohol dehydrogenase; the latter promoting the production of ethanol from aldehyde in root apexes under anaerobic conditions (Clavijo, 2001). When this mineral is coupled to catalytic enzymes, its atoms are separated from one another by chains of three amino acids; the most frequent one being histidine, followed by glutamine and asparagine. Zn atoms with structural functions are usually coordinated with S groups, together with cystein. These complexes constitute stable structures that play important roles in DNA replication and genetic expression (Coleman, 1992).
The biochemical paths involving Zn affect plants in several ways, such as protein and sugar metabolism (the latter comprising photosynthesis and the conversion of starch into sugars), auxins (a growth regulator) synthesis, pollen formation (Sharma et al., 1990) and membrane integral maintenance (Brown et al., 1987; Alloway, 2004).
Many Zn dependent enzymes play important roles in the metabolism of proteins, carbohydrates and auxins. For example, a deficiency of this element reduces the activity of carbonic anhydrase (CA), which, being present in chloroplasts and the cytoplasm, facilitates the transference of CO2/HCO3 for the photosynthetic fixation of CO2 (Marschner and Cakmak, 1989; Cakmak, 2000). Also affected by Zn deficiency, the enzyme 1,6bifosfato regulates C6 sugars in the chloroplast and the cytoplasm, where it is located; whilealdolasa promotes the transference of C3 photosynthates from chloroplasts to the cytoplasm, where it regulates metabolite flow via glycolytic processes (Marschner and Cakmak, 1989; Cakmak, 2000).
Auxin (specifically IAA) metabolism alterations are closely associated with Zn deficiency symptoms such as intervein chlorosis (which goes from green to light yellow) and short internodes (Arce et al., 1991), as well as delayed growth, small leaves, and leaf necrosis as secondary effects of P and Fe toxicity (Ramírez, 2004). If the auxin metabolism route is affected by Zn is not clear yet, but tryptophan, whose production requires this mineral, is a likely precursor of IAA synthesis. Anyway, it is clear that Zn deficiency diminishes the amount of synthesized IAA, which is, additionally, subjected to more intense oxidative degradation processes (premature tissue ageing) (Kirkby and Römheld, 2007).
Zinc deficiency is also closely related to N metabolism, in as much as it reduces the concentration of proteins and increases that of aminoacids (Kirkby and Römheld, 2007; Bell and Dell, 2008); thus, determining disease propensity through a higher exudation of these low molecular weight components (phytosiderophores) and turning over in the plant resulting from altered root and shoot growth patterns (Ramírez, 2004). There is growing evidence that the cell membrane structural integrity and permeability maintenance roles played by Zn protect the plant against the attack of pathogens on roots and new sprouts (Kirkby and Römheld, 2007; Bell and Dell, 2008).
Protein synthesis inhibition resulting from Zn deficiency is largely determined via RNA reduction, which is, in turn, provoked by a lower Zn polymerase activity, reduced structural integrity of ribosomes and higher RNA degradation. With this mineral's deficiency, the mentioned growth arrestment brings along lower carbohydrate consumption levels and, consequently, lower photosynthetic rates. This leads to a larger production of oxygen radicals, which, not being removed, intensify Zn deficiency symptoms, especially under intense luminosity (Kirkby and Römheld, 2007).
Marschner (1995) suggests that the isozyme superoxido dismutasa (SOD or Cu-Zn-SOD), which contains Zn, plays an important role in the removal of superoxidized radicals (O2-) and, therefore, in protein and membrane protection against oxidation. Zinc controls the production of free radicals, which are toxic, by interfering in NADPH oxidation and in their actual removal. Through the action of these radicals, Zinc deficiency leads to the breakage of the double bonds of phospholipids and polyunsaturated fatty acids in the cell membrane, which, in this way, becomes more permeable and tends to allow the loss of sugars, aminoacids and potassium. The damaged lipid membrane and IAA oxidation produce chlorophyll destruction and, thus, necrosis and atrophied growth of the leaves (Marschner and Cakmak, 1989).
Foliar and edaphic Zn absorption
The approximate Zn concentrations in the granitic, igneous rock and basaltic fractions of the earth's crust are, respectively, 40, 70, and 100 mg kg-1 of soil (Taylor, 1964); while sedimentary rocks such as limestone, sandstone and shale contain 16, 20 and 95 mg kg-1 of soil, respectively (Turekian and Wedepohl, 1961). The total Zn content in soils varies from 3 to 770 mg kg-1, whereas the world average is 64 mg kg-1 (Kabata-Pendias and Pendias, 1992).
Soil fertility is usually measured in terms of nutrient availability for plants. However, a soil with elevated mineral levels is not necessarily fertile because several factors, such as compaction, drainage, drought, diseases or pests, may limit nutrient availability. For this reason, the concept of fertility should also include chemical, physical and biological criteria (Pumisacho and Sherwood, 2002).
Regarding soils, Zn deficiency is caused in crops by low native levels of this element, a lack of associated minerals in the pedogenetic process, basic or calcareous reaction media, a lack of organic matter, salinity, downpouring, a loss of the arable layer due to erosion, which in turn results from steep topography or continuous tillage, and possible antagonisms between P and Fe. Although low Zn availability may occur in an ample series of soils, the deficiency of this nutrient is more thoroughly expressed in sandy soils (Alloway, 2008; Bell and Dell, 2008).
With zinc sulphate being the most frequent Zn compound employed in fertilization, other important fertilizers are: ZnEDTA (Zn chelate) and Zn nitrate (Alloway, 2008). These fertilizers can be applied to the soil or directly to the plant (foliar application). Zinc sulphate (ZnSO4 4H2O) applications to the soil range between 2 and 20 kg ha-1, while foliar applications use 0.3 to 0.5% solutions, with the highest Zn chelate translocation rates being obtained with EDTA-Zn and ZnSO4.
Foliar fertilization is actually a complement to soil fertilization. It is intended for the correction of micronutrient deficiencies and the recovery of the plant when affected by adverse biotic or abiotic conditions. The efficiency of this fertilization method is a function of crop age, foliar area, time of year, application method and mobility of the mineral in question (Pumisacho and Sherwood, 2002).
Under full foliar fertilization, potato yield has been reported to increase by 5 t ha-1. Zinc chelate applications have been found to increase yield by up to 2.6 t ha-1. Positive responses to foliar fertilization are mainly attributed to low sulphur, zinc and manganese levels. Two to four fertilizer doses at intervals of 21 d starting at flowering are usually recommended for micronutrient deficiency correction via foliar application (Pumisacho and Sherwood, 2002).
In this context, the objective of the current study was to evaluate the effects of edaphic and foliar Zn applications on the yield of the variety Criolla Colombia under the conditions of a Bogota Plateau soil. This will facilitate formulating fertilization strategies under which this plant is capable of satisfying its need for specific nutrients, such as Zn in this case, when they are not available.
Materials and methods
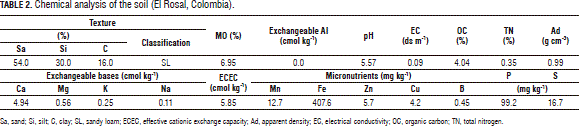
This research was conducted on the San Gabriel farm, in the municipality of El Rosal (Cundinamarca, Colombia) (2,685 m a.s.l.; with a precipitation of 825 mm year-1; annual average temperature of 13°C; and 81% relative humidity) on a Typic Hapludand loamy soil with strongly acid reaction and low cationic exchange capacity; but with good levels of Ca, probably due to the effects of previous soil amendments. Showing no aluminum limitations, it did present Mg misbalances. Native Zn was at moderate to adequate levels (4-6 mg kg-1), but the P/Zn ratio, which was above 10, might have absorbed the mineral in question. Regarding other ionic ratios, the P and Ca levels might have determined the K, Mg, B and Zn deficiencies. These analyzes were performed in the Universidad Nacional de Colombia, Soils Laboratory, Bogota (Tab. 2).
Plant material
The experiment made use of 2-4 cm seed-tubers of the cultivar Criolla Colombia, which are typically round shaped and feature medium-depth eyes, an intense yellow flesh and rind, an early maturation (120 d), a specific gravity of 1.088, no dormant period, and an average yield of 13-15 t ha-1 (Rodríguez et al., 2009).
The zinc application was intended as a complement to the conventional fertilization plan (kg ha-1): N, 88.39; P2O5, 232.09; K2O, 113.29; CaO, 56; MgO, 70.75; and S, 6. The zinc sources employed for the trial were Microzinc® (Microfertisa, Bogota) (20% granulated Zn sulphate) and EDTA- Zn chelate, 12% soluble powder.
The band application of ZnSO4 to the plot was carried out together with that of the other fertilizers. Thus, the Zn doses were 0, 1, 2 and 3 kg ha-1. Likewise, each foliar Zn dose was fractioned into five applications starting on day 30 after planting and continuing on days 37, 44, 51 and 58.
The foliar applications were carried out with a 20 L RoyalCondor® sprayer pump (Progen, Bogota). Each dose was applied with 300 L ha-1 of water. Additionally, the water was treated with MF-Acidurez® SP, 0.25 g L-1 (Microfertisa, Bogota) (hardness corrector and pH reducer) and Herbox-SL (Exro, Bogota) 0.75 cm3 L-1 (hypotensive coadjuvant) to improve the application efficiency of the element in question.
Experiment design
The experiment was carried out under a split-plot, randomized complete block design with four repetitions, considering the Zn application dose as factor A (0, 1, 2, 3 kg ha-1) and the application technique (edaphic or foliar) as factor B. The experimental units corresponded to 21.6 m2 plots with 0.9 m between furrows and 0.3 m between plants.
Studied variables
The yield was assessed in terms of number of tubers and tuber production weight in two size categories. Thus, the following variables were measured: class 1 yield (C1Y, corresponding to the weight of those tubers with a diameter larger than 4 cm); class 2 yield (C2Y, 2-4 cm tubers); and commercial yield (CY), which grouped categories 1 and 2. Regarding the number of tubers in 120 m2, NC1T represented class 1 tubers (diameter > 4 cm); NC2T, 2-4 cm diameter tubers; and NTT, classes 1 and 2. The harvest took place on day 105 after sowing.
Statistical analysis
The statistical treatment of the data consisted of an analysis of variance (ANOVA) carried out in SAS v9.0 (SAS Institute, Cary, NC) and Office Excel®Â (Microsoft Corporation, Washington DC), comparing two factors: Zn dose (0, 1, 2, 3 kg ha-1) and application technique (edaphic and foliar) with their corresponding interactions. As the data passed the normality test, each variable was further scrutinized by Bartlett's variance homogeneity test, which indicated the general fulfillment of this assumption except for the total number of tubers.
The homogeneous variables were evaluated through analysis of variance. Those showing significant differences were further analyzed through orthogonal contrasts. Finally, a tendency line was adjusted for those variables showing Zn dose effects. The variable total number of tubers (TN1) was elevated to the power of two, thus stabilizing its variances, which allowed for proceeding to the ANOVA.
Results and discussion
Provided that, except for the total number of tubers (TC), all variables showed normal behavior, we resorted to Levene and Bartlett's homogeneity tests. As this variable showed no variance homogeneity, it required a transformation to stabilize the data. The remaining variables were found to be homocedastic. As a consequence, the analysis of variance was carried out with the original data.
Class 1 yield (C1Y)
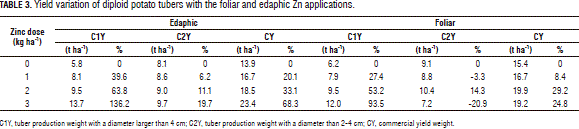
This variable revealed significant differences between Zn doses within both foliar and edaphic application methods. However, no such difference was found between them or resulting from their interaction with the mineral doses. The contrast analysis showed how this variable was positively correlated to the Zn dose for each application technique. Table 3 shows how the 3 kg ha-1 dose had a positive effect on the crop, as can be seen from the yield increase data: 7.9 t ha-1 (136%) for the edaphic application and 5.8 t ha-1 (93%) for the foliar application.
This positive effect exerted by Zn on total yield indicates a better efficiency in the growth processes and assimilate conversion, translocation and accumulation (Gómez et al., 2007). In working with the stubborn onion, Gómez et al. (2007) observed that the simultaneous application of Zn and Mn resulted in better class 1 yields (35.36 t ha-1) than those obtained without the incorporation of these micronutrients in the N P K Mg fertilization program.
Class 2 yield (C2Y)
This variable presented a linear interaction between the studied factors. That is to say, each application method had a different behavior in face of the Zn dose. Tab. 3 allows one to observe how yield was still positively correlated to the edaphic Zn dose, albeit not as strongly as in class 1. The 3 kg ha-1 Zn dose gave the best result, with a 1.9 t ha-1 yield increase (19%) with respect to the control. The foliar Zn application had a different behavior. The dose that allowed the best results was 2 kg ha-1, which resulted in an increase of 1.3 t ha-1 (14%).
This difference between the treatments was probably due to the foliar P/Zn ratio, which has been investigated by Marschner (1995) and by Khan and Ajakaiye (1976). These authors detected an antagonism between these two minerals, resulting from the fact that excessive amounts of P (P/Zn ratios above 55) reduce yield. Specifically, P excesses lead to the production of Zn metabolic disorders consisting of the synthesis of insoluble compounds containing this mineral, all of which limit its long distance absorption (Marschner, 1995). Pumisacho and Sherwood (2002), stated that, in the case of the potato, P is a critical nutrient for the initial yield and development of the plant, in as much as it promotes root growth and rapid tuber formation.
Commercial yield (CY)
As in the case of C1Y, CY only revealed significant differences between the Zn dose treatments, but not between their application methods, which exhibited a linear behavior, as in CY1. Said differences were observed within both the foliar and edaphic application techniques. Table 3 shows how the edaphic application rendered a 9.5 t ha-1 (68%) yield increase with the 3.0 kg ha-1 Zn dose, while the foliar application resulted in a 4.5 t ha-1 (29%) increase with the 2.0 kg ha-1 dose.
The observed yield increases in C1Y, C2Y and CY were probably caused by correlated photosynthesis and hormone synthesis increments. Among the latter, auxins are particularly important, as far as they participate directly in root development, in agreement with similar remarks by Gómez (2005). In addition, auxin metabolism not only promotes stem and coleoptile elongation through better solute intake and protein and polysaccharide synthesis and storage, but - as stated by Salisbury and Ross (2000) - adventitious root formation and vascular differentiation as well.
Number of class 1 tubers (NC1T)
Although the studied Zn doses had significant effects on this variable, the application techniques did not, just as they showed no mutual interaction as well, which implies that they had an independent behavior. The contrast analysis applied to this data highlights the important role played by Zn in short-cycle crops such as the diploid potato, in which this nutrient allows better structure differentiation and filling due to more efficient assimilate accumulation (Gómez et al., 2007).
The percent variation in the number of tubers revealed a positive effect of Zn fertilization on diploid potato yield (Tab. 4), as far as the greatest variations were observed in the class 1 tubers, in agreement with the NC1T and NT data. In effect, a 77% increase was observed in the class 1 tubers with the edaphic application treatment, while the foliar one resulted in a 86% increase. In contrast, the class 2 tubers were found to decrease, even to a level below that of the control under both application methods. Since commercial yield contains more class 1 than class 2 tubers, these data indicate that the plant is behaving more efficiently in terms of tuber filling.
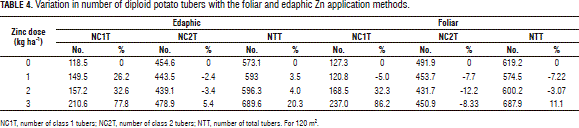
The observed increase in the number of class 1 tubers might be associated with a positive response to Mg, as also reported by Pérez et al. (2008). In evaluating this same variety, these authors suggest that Zn stimulates Mg utilization because they both play active roles in photosynthesis.
The results of the present research correspond with those of Gómez (2006), who reported a 25% yield increase with regards to the control in Zn treated stubborn onions. Similarly, Peña et al. (1999) found that 7 kg ha-1 doses of this mineral increase onion yield (Gómez et al., 2007).
Technical optimum
As the observed crop response correlates to Zn dose, its optimum was sought with a polynomial regression (Fig. 1). However, under the foliar application method, this increase was only marginal with the 3 kg ha-1 dose (Fig. 2), implying that, at elevated doses, plant yield diminishes, probably due to a phytotoxic reaction or to the P/Zn ratio. In turn, a linear response was observed under the edaphic application method (Fig. 2), which suggests that the optimum dose has to be sought by increasing the Zn application until there is a yield decline.
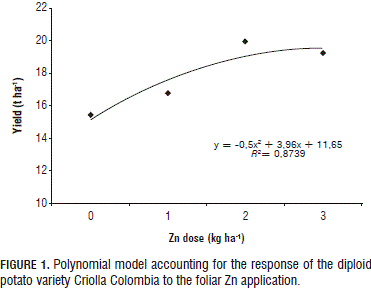
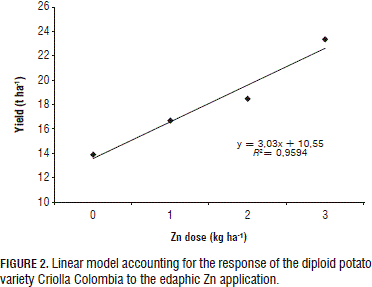
The positive response obtained in the current study with the 3 kg ha-1 dose corroborates reports by Gómez et al. (2007) and Murphy and Walash (1972), according to which the best yield response was found with the 3.5 kg ha-1 dose; therefore, resulting in a recommended Zn dose of 3.4 to 4.5 kg ha-1.
Conclusions
Zinc is an important nutrient, as far as it enhances crop efficiency by improving both P absorption and size and weight quality.
The positive response obtained in the current foliar and edaphic Zn application trial could be fruitfully applied by diploid potato growers on the Bogota Plateau in order to optimize crop yield. However, a comprehensive evaluation of plant nutrition in this case calls for an assessment of dry weight productivity and Zn interaction with other elements.
The application technique is of great interest, as far as it is part of an integrated plant nutrition management strategy. Although the edaphic method is the most frequent, economical and efficient one, foliar applications are also attractive after considering the series of soil factors that limit Zn absorption, which make them a means of quick micronutrient supply.
Based on the linear behavior exhibited by the edaphic Zn fertilization, future research should aim at determining an optimum Zn application level by taking into consideration marginal points and further elevated doses, so as to find toxicity levels, which are observable through yield decreases.
Acknowledgements
The authors express their gratitude to Manuel Caicedo, General Manager of Sociedad Agraria de Transformación (Agrarian Society for Transformation at El Rosal, Cundinamarca), for his collaboration and to Microfertisa for its technical and economic support.
Literature cited
Alloway, B. 2004. Zinc deficiency in crops: causes and corrections. Department of Soil Science, The University of Reading, Reading, UK. [ Links ]
Alloway, B.J. 2008. Zinc in soils and crop nutrition. 2nd ed. International Zinc Association (IZA); International Fertilizer Industry Association (IFA), Brussels. [ Links ]
Arce, J.P., B. Storey, and C.G. Lyons. 1991. Effectiveness of three different Zn fertilizers and two methods of application for the control of 'little-leaf' in peach trees in South Texas. HortScience 26(5), 497. [ Links ]
Becerra-Sanabria, L.A., S.L. Navia-de Mosquera, and C.E. Ñústez-López. 2007. Efecto de diferentes niveles de fósforo y potasio sobre el rendimiento del cultivar "Criolla Guaneña" en el departamento de Nariño. Rev. Latinoam. Papa 14(1), 51-60. [ Links ]
Brown, P.H., R.M. Welch, and E.E. Cary. 1987. Nickel: a micronutrient essential for higher plants. Plant Physiol. 85(3), 801-803. [ Links ]
Cakmak, I. 2000. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 146, 185-2005. [ Links ]
Clavijo, J. 2001. Metabolismo de los nutrientes en las plantas. pp. 11-28. In: Silva M., F. (ed.). Fertilidad de suelos, diagnóstico y control. 2nd ed. Sociedad Colombiana de la Ciencia del Suelo (SCCS), Bogota. [ Links ]
Bell, R.W. and B. Dell. 2008. Micronutrients for sustainable food, feed, fibre and bioenergy production. International Fertilizer Industry Association (IFA), Paris. [ Links ]
Coleman, J.E. 1992. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 61, 897-946. [ Links ]
Hawkes, J.G. 1990. The potato: evolution, biodiversity and genetic resources. Belhaven Press, Smithsonian Institution Press, London, Washington DC. [ Links ]
Fedepapa, Federación Colombiana de Productores de Papa. 2012. Indicadores 2012. Rev. Papa 25, 45-46. [ Links ]
Herrera, A.O. and L.E. Rodríguez. 2012. Tecnologías de producción y transformación de papa criolla. Facultad de Agronomía, Universidad Nacional de Colombia, Bogota. [ Links ]
Huamán, Z. and D.M. Spooner. 2002. Reclassification of landrace populations of cultivated potatoes (SoÂlanum sect. Petota). Amer. J. Bot. 89(6), 947-965. [ Links ]
Gómez, M. 2005. Guía técnica para el manejo nutricional de los cultivos: diagnostico, interpretación y recomendación de planes de fertilización. Microfertisa, Bogota. [ Links ]
Gómez, M. 2006. Manual técnico de fertilización de cultivos. Microfertisa, Bogota. [ Links ]
Gómez, M., H. Castro, C. Gómez, and F. Gutiérrez. 2007. Optimización de la producción y calidad en cebolla cabezona (Allium cepa) mediante el balance nutricional con magnesio y micronutrientes (B, Zn y Mn), Valle Alto del rio Chicamocha, Boyacá. Agron. Colomb. 25(2), 339-348. [ Links ]
Castro, H. and M.I. Gómez, M. 2010. Fertilidad de suelos y fertilizantes. pp. 217-303. In: Burbano O., H. and F. Silva M. (eds.). Ciencia del suelo. Principios básicos. Sociedad Colombiana de la Ciencia del Suelo (SCCS), Bogota. [ Links ]
Kabata-Pendias, A. and H. Pendias. 1992. Trace elements in soils and plants. 2nd ed. CRC Press, Boca Raton, FL. [ Links ]
Kirkby, E. and V. Römheld. 2007. Micronutrientes na fisiologia de plantas: funções, absorção e mobilidade. Informações Agronômicas No. 118. Internacional Plant Nutrition Institute (IPNI), Norcross, GA. [ Links ]
Kochian, L. 1991. Mechanism of micronutrient uptake and translocation in plants. pp. 229-296. In: Mortvedt, J.J., F.R. Fox, L.M. Shuman, and R.M. Welch (eds.). Micronutrients in agriculture. Book Series No. 4. Soil Science Society of America, Madison, WI. [ Links ]
Marschner, H. 1995. Mineral nutrition of higher plants. 2nd ed. Academic Press, San Diego, CA. [ Links ]
Marschner, H. and I. Cakmak. 1989. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium and magnesium deficient beans (Phaseolus vulgaris) plants. J. Plant Physiol. 134(3), 308-315. [ Links ]
Martínez, H., N. Pinzón, and C. Barrios. 2006. La cadena de la papa en Colombia. Una mirada global de su estructura y dinámica 1991-2005. Observatorio Agrocadenas Colombia, Ministerio de Agricultura y Desarrollo Rural (MADR); Inter-American Institute for Cooperation on Agriculture (IICA), Bogota. [ Links ]
Murphy, L.S. and L. M. Walsh. 1972. Correction of micronutrient deficiencies with fertilizers. pp. 371-381. In: Micronutrientes in agriculture Zn, Fe, B, Mo, Cu, Mn. Soil Science Society America, Madison, WI. [ Links ]
Pérez, L.C., L.E. Rodríguez, and M.I. Gómez. 2008. Efecto del fraccionamiento de la fertilización con N, P, K y Mg y la aplicación de los micronutrientes B, Mn y Zn en el rendimiento y calidad de papa criolla (Solanum phureja) variedad Criolla Colombia. Agron. Colomb. 26(3), 477-486. [ Links ]
Porras, P. 2005. Problemática general del sistema productivo de papa con énfasis en fisiología y manejo de suelos. pp. 1-4. In: I Taller Nacional sobre Suelos, Fisiología y Nutrición Vegetal en el Cultivo de Papa. Centro Virtual de Investigación de la Cadena Agrolimentaria de la Papa (Cevipapa), Bogota. [ Links ]
Pumisacho, M. and S. Sherwood. 2002. El cultivo de la papa en Ecuador. Instituto Nacional de Investigaciones Agropecuarias (INAP); International Potato Center (CIP), Quito. [ Links ]
Ramírez, C. 2004. Nutrición mineral y producción vegetal. pp. 57-68. In: Manejo productivo, poscosecha y exportación en fresco de hierbas aromáticas culinarias, temporada 2004-2005. Universidad Nacional de Colombia, Bogota. [ Links ]
Rodríguez, L.E., C. Ñústez, and N. Estrada. 2009. Criolla Latina, Criolla Paisa y Criolla Colombia, nuevos cultivares de criolla para el departamento de Antioquia (Colombia). Agron. Colomb. 23(3), 287-301. [ Links ]
Rodríguez, F., M. Ghislain, A.M. Clausen, S.H. Jansky, and D.M. Spooner. 2010. Hybrid origins of cultiÂvated potatoes. Theor. Appl. Genet. 121, 1187-1198. [ Links ]
Salisbury, F. and W. Ross. 2000. Fisiología de las plantas. Células: agua, soluciones y superficies. Thomson Editors Spain; Paraninfo, Madrid. [ Links ]
Sharma, P.N., C. Chatterjee, S.C. Agarwala, and C.P. Sharma. 1990. Zinc deficiency and pollen fertility in maize (Zea mays). Plant Soil 124, 221-225. [ Links ]
Spooner, D.M., J. Núñez, G. Trujillo, M. del R. Herrera, F. Guzmán, and M. Ghislain. 2007. Extensive simple sequence repeat genotyping of potato landraces supports a major reevaluation of their gene pool structure and classification. PNAS 104, 19398-19403. [ Links ]
Taylor, S. 1964. Abundance of chemical elements in the continental crust: a new table. Geochim. Cosmochim. Acta 28, 1273-1286. [ Links ]
Turekian, K. and K. Wedepohl. 1961. Distribution of the elements in some major units of the earth's crust. Geol. Soc. Amer. Bull. 72(2), 175-192. [ Links ]
Vallee, B. and D. Auld. 1990. Zinc coordination, function and structure of zinc enzymes and other proteins. Biochemistry 29, 5647-5659. [ Links ]
Vallee, B. and K. Falchuk. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73, 79-118. [ Links ]













