Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.32 no.2 Bogotá May/Aug. 2014
https://doi.org/10.15446/agron.colomb.v32n2.38287
http://dx.doi.org/10.15446/agron.colomb.v32n2.38287
1 Technical Department, INTEROC. Cota (Colombia).
2 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogota (Colombia). gfischer@unal.edu.co
3 Advisory of Research Projects. Bogota (Colombia).
Received for publication: 30 May, 2013. Accepted for publication: 30 July, 2014.
ABSTRACT
The purple passion fruit is propagated by seeds, but factors, such as hardiness and impermeability of the testa and salinity and pH of the soil, give rise to problems in germination and uniformity of seedlings. The objectives of the study were to evaluate the effect of different NaCl concentrations (0, 30, 60, 90, and 120 mM, corresponding to 0.8, 3.0, 6.0, 9.0, and 12.2 dS m-1) on the germination and emergence of purple passion fruit seeds. For the germination test, 50 seeds per Petri dish were used, which were watered with a saline solution weekly. A seed was considered germinated when the radicle reached 2 mm. In the case of seedling emergence, 50 seeds were sown in cleaned river sand at a 1 cm depth on polystyrene trays, covered with transparent plastic film. They were irrigated weekly with different NaCl concentrations and the electrical conductivity (EC) of the substrate was measured. A seedling was considered emerged when the hypocotyl was fully erect. The results showed significant differences, with germination being higher in seeds treated with 30 mM NaCl than in the control seeds, and no statistical differences for the 60 and 90 mM NaCl treatments. The emergence was significantly higher in the 0 (0.05 dS m-1 of the substrate) and 30 mM NaCl (0.71 dS m-1) treated seeds when compared with 60 mM (1.25 dS m-1), 90 mM (1.69 dS m-1) and 120 mM NaCl (2.30 dS m-1 of the substrate). There was a decline in the chlorophyll contents of the seedling cotyledons and an increased substrate EC with increasing NaCl concentrations.
Key words: propagation, seedlings, salt stress, radicle, hypocotyl, chlorophyll index.
RESUMEN
La gulupa se propaga mediante semilla, pero factores como la dureza e impermeabilidad de la testa, o edáficos como salinidad o pH del suelo, originan problemas de germinación y uniformidad de las plántulas. Con el objeto de evaluar la adición de NaCl en concentraciones de 0, 30, 60, 90, 120 mM, correspondiente a 0,8; 3,0; 6,0; 9,0 y 12,2 dS m-1, se midió su efecto en germinación y emergencia de la semilla de gulupa. Para la evaluación de la germinación se usaron 50 semillas por caja de Petri y se regaron hasta humedecer el papel filtro con las soluciones salinas semanalmente. Se consideró una semilla germinada cuando la radícula tuvo 2 mm. Para estudiar emergencia se sembraron 50 semillas a 1 cm de profundidad, en bandeja de icopor, llena de arena lavada de rio y cubierta con plástico transparente, haciendo semanalmente los riegos de NaCl por tratamiento y a su vez midiendo la conductividad eléctrica (CE) del sustrato. Se consideró una plántula emergida cuando el hipocótilo estuvo completamente erecto. Se encontraron diferencias significativas en germinación, siendo mayor en las semillas tratadas con 30 mM de NaCl que las del control, sin diferencias estadísticas con las de 60 y 90 mM de NaCl. La emergencia fue significativamente más alta en semillas regadas con 0 (0,05 dS m-1 del sustrato) y 30 mM de NaCl (0,71 dS m-1) en comparación con 60 mM (1,25 dS m-1), 90 mM (1,69 dS m-1) y 120 mM NaCl (2,30 dS m-1 del sustrato). Se presentó una disminución de clorofilas en cotiledones de las plántulas y aumento de la CE del sustrato a medida que aumentó la concentración de NaCl.
Palabras clave: propagación, plántulas, estrés por salradícula, hipocótilo, índice de clorofila.
Introduction
Passiflora cultivation in Colombia is of great importance because it represents an important line in the fruit production sector (Jiménez et al., 2012). Also, this genus has a lot of diversity, which allows for providing a wide range of plants for the domestic and international markets.
Purple passion fruit seeds are considered orthodox (Costa et al., 1974; IBPGR, 1985), implying that they can be dried to a 6-8% moisture content without affecting their viability and storage potential. The emergence of seedlings is phanerocotylar and epigeal. Passiflora sp. seed exogenous dormancy probably has combined mechanical and chemical dormancies (Miranda et al., 2009). They usually germinate between 20 and 30 d (Morton, 1987), with a germination percentage of 80-85%. In the field, the seedling emergence from fresh passion fruit seeds starts between 11 and 12.5 d after sowing (Miranda et al., 2009). In general, the germination of Passiflora sp. seeds is slow (Delanoy et al., 2006; Meza et al., 2007).
Taiz and Zeiger (2010) described two components of salinity stress (mostly high Na+ and Cl- concentrations) which are nonspecific (osmotic stress that causes water deficits) and specific (accumulation of toxic ions that disturb nutrient acquisition and that are cytotoxic (Munns and Tester, 2008).
Salt stress induces various morphological, physiological and biochemical responses from plants, depending on the genotype and the stage of plant development (Willadino and Camara, 2003). Plant growth is retarded by physiological processes, such as photosynthesis, stomatal conductance, osmotic adjustment, ion uptake, protein synthesis, nucleic acid synthesis, enzymatic activity, and hormonal balance (Parés et al., 2008). It also affects the transport process of water and ions, resulting in ion toxicity and nutritional imbalance (Larcher, 2003) and, consequently, vegetative growth variables, such as dry mass, plant height, and leaf area, are severely affected (Parés et al., 2008).
Plants exposed to saline conditions are affected from germination to more advanced stages of development. In the case of seeds, saline conditions slow down seed imbibition and, hence, there is a decrease in the speed of germination, because of the osmotic effect. The processes of cell division and elongation may also have abnormalities as well as the mobilization of reserves necessary for the germination process (González and Ramírez, 1996). In general, NaCl could affect seed germination in several ways: 1) generate enforced seed dormancy, such as in Atriplex prostrata (Chenopodiaceae), a salt tolerant plant species (Khan et al., 2003), 2) provoke a delay in germination and a slow rate of germination (Kudred and Sener, 1990), and 3) cause seed death. In seeds of halophyte species, secondary dormancy could be induced when seeds are exposed to 1% NaCl (Ungar, 1991).
In the passion fruit, Meza et al. (2007) observed that, at increasing salt concentrations of NaCl and KIO3 the germination percentage was significantly and negatively affected. Emergence initiation and the time that elapses for the period of 10 to 90% emergence were unaffected; whereas, the percentage of total emergence tended to decrease significantly with an increasing total concentration of salts (Meza et al., 2007). According to Nyagah and Musyimi (2009), sodium chloride solutions reduced radicle growth and plumule growth in passion fruit seedlings. There was no seed germination at NaCl concentrations of 3.6, 5.4, or 7.2 dS m-1.
The seed tolerance to salinity in germination is an ability to withstand the effects of high concentrations of soluble salts in the medium (Taiz and Zeiger, 2010; Goykovic and Saavedra, 2007). The presence of salts in the medium decreases water potential, which results in a reduced availability of water for the seeds, so seeds must generate a sufficient osmotic potential to improve the water status of embryos and allow growth (Jones, 1986).
Years ago, it was found that the majority of plants are more sensitive to salinity processes during germination and emergence than during the subsequent stages of growth and development (Ayers, 1950). Although there are several studies related to the effect of salt on different stages of development in Passifloraceae, in the purple passion fruit, this aspect has been little studied, that is, the tolerance to salinity at the early stages of growth and development of this plant is unknown.
Materials and methods
This research was conducted in the Laboratory and greenhouses of Plant Physiology at the Faculty of Agronomy of the Universidad Nacional de Colombia, Bogota. Purple passion fruit seeds extracted from fruits at maturity stage 3 were used (Pinzón et al., 2007). Thirty fruits were used in the extraction of the seeds and the pulp was allowed to ferment for 4 d; then, the seeds were washed and allowed to dry for 4 d. The seeds were sterilized for 10 s with 5% sodium hypochlorite and, then, washed with distilled water.
Germination
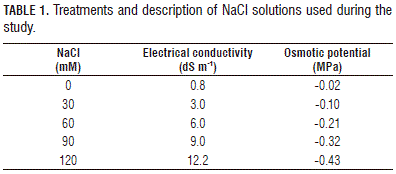
For the evaluation of germination, 50 seeds were placed in Petri dishes and watered to moisten the filter paper with different concentrations of NaCl (0, 30, 60, 90, or 120 mM) (Tab. 1). Then, the Petri dishes were taken to a growth chamber with temperatures of 23/20°C day/ night, 80% relative humidity and 12 h light (Sanyo Versatile test Environment Chamber-Kasay, Ettern Leur, The Netherlands). Once a week, the moistened filter paper was changed, according to each treatment, and the counting of germinated seeds was done every 4 d, starting from the baseline. A seed was considered germinated when a radicle length of 2 mm was obtained.
Emergence
In the greenhouses, emergence tests were conducted. For this purpose, 20x20x5 cm polystyrene trays and cleaned river sand substrate were used. Fifty purple passion fruit seeds were planted per treatment at a depth of 1 cm and covered with a transparent plastic. The average temperature and relative humidity under the plastic cover were 16.8°C and 76%, respectively. The trays were watered once a week with NaCl salt solutions, according to the treatment (Tab. 1), ensuring that they were watered at near field capacity with additional irrigation with distilled water. The electrical conductivity (EC) of the substrate was measured weekly (Konduktometer Schott, Schott Geräte GmbH, Hofheim, Germany). Normal seedlings were considered as those that showed the potential for further development and gave rise to healthy plants when transplanted to a good soil and favorable environmental conditions. A seedling was considered emerged when the hypocotyl was fully erect. This factor was measured every 4 d from the start of the experiment.
Measurement of imbibition, electrical conductivity, and chlorophyll content
At the baseline, the imbibition of the purple passion fruit seeds was assessed, with four seeds as a replicate taken for each treatment, from which the weights were obtained and averaged for each treatment. This measurement was performed every 4 d before the first seed germination. The rationale for this measurement was to show how the concentration of the NaCl solution affected the water absorption of the seeds and what impact this had on the germination.
The measurement of the chlorophyll index content (SPAD units) was done with Minolta SPAD equipment at the end of the experiment. For this, in each treatment, two emerged seedlings were taken per replicate and the chlorophyll content index was measured directly in the cotyledon of each seedling, obtaining an average per treatment.
Experimental design
The design used a randomized complete block with five treatments and five replicates, for a total of 50 experimental units, using a Petri dish (germination) and polystyrene tray with cleaned sand (emergence) as the experimental unit and the number of sampling times as blocks. The imbibition and EC data were analyzed using sampling as a replicate.
Verification tests were performed on assumptions for parametric analysis and processing of data for the variables that showed lack of normality. Once parametric assumptions of the evaluated variables were verified, descriptive statistics and analysis of variance, using the SAS® 9.0 statistical software (SAS Institute, Cary, NC), were carried out. For variables that showed evidence of significant differences, a Tukey's multiple comparison test was used. In all tests, a significance level of (P≤0.05) was used.
Results and discussion
Imbibition and germination
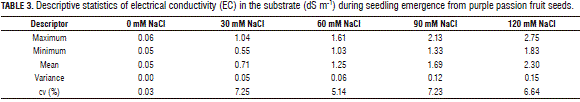
Seed imbibition was measured until 16 DAS (days after sowing), before germination, during which five samplings were taken to measure seed weight per treatment. In seed imbibition, there were no significant differences between the treatments for seed weight, using Anova. However, when performing data descriptive statistics (Tab. 2), the control and 30 mM NaCl treatment had the higher weight variation coefficients, at 10 and 9%, respectively, and the other treatments had a variation exceeding 6%. This shows that, with an increasing NaCl concentration, the process of seed imbibition decreased because the osmotic potential decreased in the growth medium and, hence, the water potential decreased (Martínez et al., 2011; Zekri, 2002), represented in the seed weight due to water absorption.
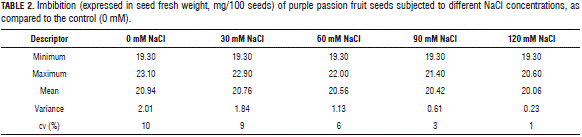
According to our data, the treatment with the highest imbibition was the control, with a maximum of 23.10 mg, and the lowest weight was in the 120 mM NaCl treatment, with 20.6 mg, showing a trend in decreasing weight in inverse proportion to the NaCl concentration in the medium. However, the average values of all treatments were found in a range of from 20 to 21 mg in weight, which was not statistically different.
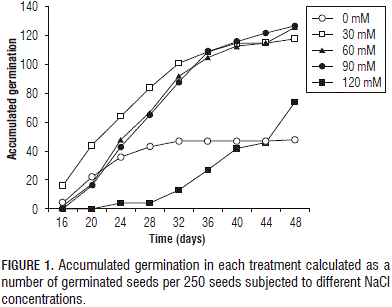
Figure 1 shows the cumulative germination in the treatments with different concentrations of NaCl in nine samples, performed at 48 d after placement in the Petri dishes. The germination presented sigmoid curves over time for each of the salinity treatments. The germination started at 16 DAS with the 0, 30, and 60 mM NaCl treatments and later for the 90 to 120 mM NaCl treatments, at 20 and 24 DAS , respectively. These results indicate that, as the NaCl concentration increased, there was a decrease in germination.
After performing the analysis of variance, with germination data transformed by the square root function, there was a highly significant difference (P≤0.0001) between the treatments, showing a higher germination for the treatments of 60, 30, and 90 mM NaCl than the control, with the lowest at 120 mM (Fig. 2).
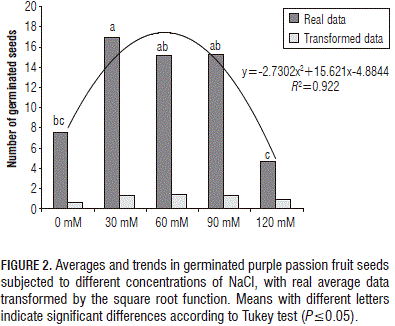
According to Larcher (2003), germination is more successful in salt-free settings or in those having extremely low saline conditions , but, in our experiment, the purple passion fruit seeds in the 30, 60 and 90 mM NaCl concentrations did not germinate any differently than in the control, especially after 24 d, confirming the germination tolerance of this species to low and moderate salt concentrations as Miranda et al. (2010) observed in the cape gooseberry (Physalis peruviana).
Figures 1 and 2 show important reductions in germination rates in the purple passion fruit seeds subjected to the 120 mM NaCl concentration. In this case, Miranda et al. (2010) supposed that the seed osmotic adjustment was affected and that stress had possibly favored the entering of other ions into the seeds. The low humidity content of the seeds may have increased saline stress, causing a cessation of metabolism or inhibition of certain stages in the germination metabolic sequence (Smith and Comb, 1991).
Meza et al. (2007), in Passiflora edulis f. flavicarpa, observed the same trend and found that the germination decreased with increased salt concentrations. Studies in Brazil, using eight water EC levels ((ECw)) of between 1 and 8 dS m-1, showed that the seeds of passion fruits are moderately tolerant to salinity in terms of vigor and initial development of the plants (Loureiro et al., 2002). However, (ECw) of from 4.43 dS m-1 showed adverse effects on the germination of this species.
Studies, in which seeds of various cultivars of Solanum lycospersicum were treated with increasing concentrations of NaCl, have shown that the germination rate decreased with increasing salinity and the germination period took longer (Singer, 1994; El-Habbasha et al., 1996; Cuartero and Fernández-Muñoz, 1999).
In this study, the germination was slow, since, in the best of cases, it reached 50% in 48 DAS (Fig. 1), demonstrating that the purple passion fruit seed germination was delayed. Cárdenas (2011) mentioned that Passifloraceae seed germination is slow. Delanoy et al. (2006) reported that curuba seeds began to germinate 9 d after the beginning of the trial and reached 50% germination after 1 month. However, the period and the germination percentage varied considerably according to the conditions under which the test was performed (Aular et al., 1996; Meza et al., 2007).
It is noteworthy that the results of seed imbibition (Tab. 3) might have had a direct effect on the duration of the germination process and that, at concentrations above 60 mM NaCl, there was a substantially reduced variation coefficient of seed weight (imbibition). This might have reduced the osmotic and water potentials in the Petri dishes, affecting normal water uptake in seeds, and was reflected in the final percentage of germination, as shown by Meza et al. (2007) and González and Ramírez (1996). NaCl inhibits germination, not only due to the physiological drought caused by the reduced water potential in the medium, but also due to an increasing concentration of toxic ions in the embryo (Prisco and O'Leary, 1970).
Electrical conductivity and emergence
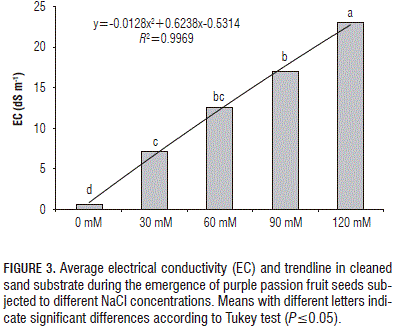
Analyzing the EC values of the cleaned sand substrate from the beginning, it was observed that there was an increase in EC as the NaCl concentration increased (Fig. 3). The Anova evidenced significant differences between the treatments (P≤0.0001) for EC of the substrate, showing that 120 mM NaCl had the highest EC as compared to the other treatments that had an average value of 2.30 dS m-1. The control had the lowest EC compared to the other treatments, with a value of 0.05 dS m-1. The 60 and 90 mM NaCl treatments differed significantly from each other, showing values of 1.25 and 1.69 dS m-1, in addition, the 60 and 30 mM NaCl treatments had values of 1.25 and 0.71 dS m-1, respectively (Fig. 3). The general trend of EC in the substrate was to increase in accordance with the NaCl concentration (Fig. 4 and Tab. 3).
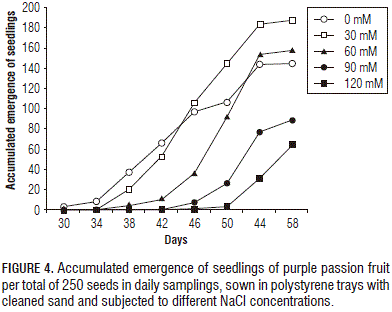
The general trend during the emergence matched a simple sigmoidal curve, with a slow phase at the beginning followed by a fast phase that varied according to the treatment (Fig. 4). In other words, the beginning of germination for the 0 mM NaCl treatment occurred at 30 DAS , at 38 DAS for the 30 and 60 mM NaCl treatments, and at 46 DAS for the 90 and 120 mM NaCl treatments, which affected the emergence percentage at 58 DAS , when they reached the maximum percentage of germination.
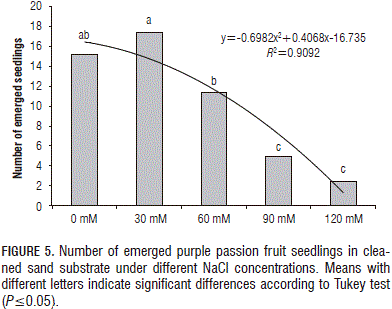
Analysis of variance of the emergence showed a highly significant difference (P≤0.0001) between the treatments, with the treatments of 30 and 0 mM NaCl having the better emergences, while the treatments of 90 and 120 mM NaCl had the lower emergences. The treatments of 60 and 0 mM NaCl did not present differences (Fig. 5).
Increasing NaCl concentrations in the substrate caused a negative effect on the emergence percentage and rate of the purple passion fruit seeds, which was more noticeable starting at thirty days after planting. Some authors have reported the same, such as Meza et al. (2007), who studied passion fruit seeds sown in coconut fiber and cleaned river sand at a 2:1 ratio with different levels of salinity, finding significant differences between the treatments.
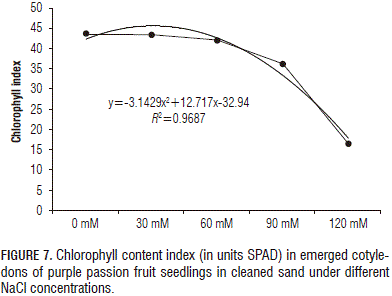
Salinity produced an appreciable effect on the duration of the emergence, which was, ultimately, negative, i.e. the total emergence had a inversely proportional trend to the increase in NaCl concentration , especially when it exceeded 90 mM in the substrate (Figs. 6 and 7). In this regard, Zekri (2002) reported that the emergence in Citrus sp. seeds decreased directly proportional to increasing salinity levels, noting that this trend was less consistent in 'Cleopatra' mandarin because this rootstock exhibited a higher salt tolerance.

Hadas (1977) reported that the germination process is inhibited from the time the seed is not able to take up water by imbibition, causing delays in the metabolic activation necessary for the emission of the radicle and subsequent mobilization of reserves in different seed parts (Jamil et al., 2006), and by excessive absorption of toxic ions, specifically Na+ and Cl- (Prisco and O'Leary, 1970). Therefore, a positive relationship can be seen between germination and seedling emergence, which is demonstrated by the data in this study, which show that the increasing NaCl concentration (in the Petri dishes and in the substrate) not only affected the germination percentage but also negatively impacted the emergence of the seeds, which may indicate the toxic effects of NaCl.
Chlorophyll in cotyledons
The measurement of chlorophyll content in the cotyledons at the end of the experiment indicated that, as the NaCl concentration increased in the substrate, there was a clear decrease in the index of chlorophyll content (Fig. 7).
Some studies have shown that salinity affects the chlorophyll content in plants of agricultural interest. Argentel et al. (2009) determined the chlorophyll content and ion accumulation in various organs of wheat plants of the variety Cuban-C-204 by applying different levels of NaCl, adjusted to 4.8 and 12 dS m-1; they found a decrease in the chlorophyll contents as the salt concentrations increased, which was significant only between the control (0.02 dS m-1) and the 12 dS m-1 treatment; the chlorophyll content decreased significantly when the EC exceeded 8 dS m-1.
Studies on the effect caused by salinity on the photosynthetic pigment concentration are abundant and coincident and tend to show that the damage is mainly due to the destruction of chloroplasts and increased activity of the enzyme chlorophyllase, affecting the synthesis of chlorophyll (Flowers and Yeo, 1986; Appels and Lagudah, 1990).
Conclusions
Increasing NaCl concentrations significantly affected the accumulated germination and the percentage of germination in the purple passion fruits, especially at the higher levels of 90 and 120 mM, equivalent to 9.0 and 12.2 dS m-1, respectively.
The NaCl concentration in the substrate had significant differences for the EC, showing a proportional trend between treatments, being higher for the 120 mM NaCl treatment with 2.3 dS m-1 and lower for the control with 0.05 dS m-1.
The emergence of purple passion fruit seedlings was significantly affected by increasing salinity levels in the substrate, with the lower percentages of emergence occurring with the 90 and 120 mM NaCl treatments, equivalent to 1.69 and 2.3 dS m-1 in the substrate, respectively.
The different NaCl concentrations in the substrate had negative effects on the chlorophyll content in the seedlings, showing a clear decrease with increased NaCl concentrations, with the lowest rate at 120 mM.
The ability to germinate and emerge under low saline stress conditions could indicate that the purple passion fruit possesses a genetic potential for salt tolerance, at least during the first developmental stages. According to Miranda et al. (2010), this behavior does not necessarily suggest that plantlets initiated in saline stress conditions can continue to grow and complete their adult plant life cycle under these conditions.
Literature cited
Appels, A. and H.E. Lagudah. 1990. Manipulation of chromosomal segments from wild wheat for the improvement of breadwheat. Aust. J. Plant Physiol. 17, 253-266. [ Links ]
Argentel, L., D.R. López, L.M. González, R.C. López, E. Gómez, R. Girón, and I. Fonseca. 2009. Contenido de clorofila e iones en la variedad de trigo harinero Cuba-c-204 en condiciones de estrés salino. Cultivos Trop. 30, 32-37. [ Links ]
Aular, J., A.D. Bautista, and N. Maciel. 1996. Influencia de la luz, la profundidad de siembra y el almacenamiento sobre germinación y emergencia de la parchita. Agro. Trop. 46, 73-83. [ Links ]
Ayers, A.D. 1950. Seed germination as affected of soil moisture and salinity. Agron. J. 44, 82-84. [ Links ]
Cárdenas, J. 2011. Morfología y tratamientos pre-germinativos de semillas de granadilla (Passiflora ligularis Juss.). M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota. [ Links ]
Costa, C.F. da, E.L.P.G. de Oliveira, and W.T. Lellis. 1974. Persistence of the germinating capacity of passion fruit seeds. Boletim do Instituto Biologico da Bahia 13, 76-84. [ Links ]
Cuartero, J. and R. Fernández-Muñoz. 1999. Tomato and salinity. Sci. Hortic. 78, 83-125. [ Links ]
Delanoy, M., P. Van Damme, X. Scheldeman, and J. Beltran. 2006. Germination of Passiflora mollissima (Kunth) L.H. Bailey, Passiflora tricuspis Mast and Passiflora nov sp. seeds. Sci. Hortic. 110, 198-203. [ Links ]
El-Habbasha, K.M., A.M. Shaheen, and F.A. Rizk. 1996. Germination of some tomato cultivars as affected by salinity stress condition. Egyp. J. Hort. 23, 179-190. [ Links ]
Flowers, T.J. and A.R. Yeo. 1986. Ion relations of plants under drought and salinity. Aust. J. Sci. Res. 13, 75-91. [ Links ]
González, L. and R. Ramírez. 1996. Respuesta de Terannus labilis a diferentes niveles de salinidad durante la germinación y crecimiento. Cultivos Trop. 17, 17-19. [ Links ]
Goykovic, V. and G. Saavedra. 2007. Algunos efectos de la salinidad en el cultivo del tomate y prácticas agronómicas de su manejo. Idesia 25, 47-58. [ Links ]
Hadas, A. 1977. Water uptake and germination of leguminous seeds in soils of changing matrix and osmotic water potential. J. Exp. Bot. 28, 977-985. [ Links ]
IBPGR. 1985. Passifloraceae. In: Ellis, R.H., T.D. Hong, and E.H. Roberts (eds.). Handbook of seed technology for genebanks. Vol. 2: Compendium of specific germination information and test recommendations. Intl. Plant Genet. Resources Inst., Rome. [ Links ]
Jamil, M., D.B. Lee, K.Y. Jung, A. Muhammad, S.CH. Lee, and E.S. Rha. 2006. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. J. Centr. Europ. Agr. 7, 273-282. [ Links ]
Jiménez, Y., C. Carranza, and M. Rodríguez. 2012. Gulupa (Passiflora edulis Sims.). pp. 579-599. In: Fischer, G. (ed.). Manual para el cultivo de frutales en el trópico. Produmedios, Bogota. [ Links ]
Jones, R.A. 1986. High salt tolerance potential in Lycopersicon species during germination. Euphytica 35, 575-582. [ Links ]
Khan, M.A., I.A. Ungar, and B. Gul. 2003. Alleviation of salinityenforced seed dormancy in Atriplex prostrata. Pak. J. Bot. 35, 906-912. [ Links ]
Kudred, K. and B. Sener. 1990. Effects of kinetin and gibberellic acid in overcoming high temperature and salinity (NaCl) stresses on the germination of barley and lettuce seeds. Phyton 30, 65-74. [ Links ]
Larcher, W. 2003. Physiological plant ecology. 4th ed. Springer- Verlag, Berlin. [ Links ]
Loureiro, F., H. Gheyi, S. Batista, C. Uyeda, and P. Fernandes. 2002. Water salinity and initial development of yellow passion fruit. Sci. Agric. 59, 491-497. [ Links ]
Martínez, N., C.V. López, M. Basurto, and R. Pérez. 2011. Efectos por salinidad en el desarrollo vegetativo. Tecnociencia 5, 156-161. [ Links ]
Meza, N., M. Arizaleta, and D. Bautista. 2007. Efecto de la salinidad en la germinación y emergencia de semillas de parchita (Passiflora edulis f. flavicarpa). Rev. Fac. Agron. (LUZ) 24, 69-80. [ Links ]
Miranda, D., M. Perea, and S. Magnitskiy. 2009. Propagación de especies pasifloráceas. pp. 69-96. In: Miranda, D., G. Fischer, C. Carranza, S. Magnitskiy, F. Casierra, W. Piedrahíta, and L.E. Flórez (eds.). Cultivo, poscosecha y comercialización de las pasifloráceas en Colombia: maracuyá, granadilla, gulupa y curuba. Sociedad Colombiana de Ciencias Hortícolas, Bogota. [ Links ]
Miranda, D., Ch. Ulrichs, and G. Fischer. 2010. Imbibition and percentage of germination of cape gooseberry (Physalis peruviana L.) seeds under NaCl stress. Agron. Colomb. 28, 29-35. [ Links ]
Morton, J.F. 1987. Fruits of warm climates. Creative Resources Systems, Winterville, NC. [ Links ]
Munns, R. and M. Tester. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651-681. [ Links ]
Nyagah, A.W. and D.M. Musyimi. 2009. Effects of sodium chloride solution on germination and growth of fruits seedlings. ARPN J. Agric. Biol. Sci. 4, 49-53. [ Links ]
Parés, J., M. Arizaleta, M.E. Sanabria, and G. García. 2008. Efecto de los niveles de salinidad sobre la densidad estomática, índice estomático y el grosor foliar en plantas de Carica papaya L. Acta Bot. Venez. 31, 27-34. [ Links ]
Pinzón, I.M.P., G. Fischer, and G. Corredor. 2007. Determinación de los estados de madurez del fruto de la gulupa (Passiflora edulis Sims). Agron. Colomb. 25, 83-95. [ Links ]
Prisco, J.T. and J.W. O'Leary. 1970. Osmotic and "toxic" effects of salinity on germination of Phaseolus vulgaris L. seeds. Turrialba 20, 177-184. [ Links ]
Singer, S.M. 1994. Germination responses of some tomato genotypes as affected by salinity and temperature stress. Egyp. J. Hort. 21, 47-64. [ Links ]
Smith, P.T. and B.G. Comb. 1991. Physiological and enzymatic activity of pepper seeds (Capsicum annuum) during priming. Physiol. Plant. 82, 71-78. [ Links ]
Taiz, L. and E. Zeiger. 2010. Plant physiology. 5th ed. Sinauer Associates Publishers, Sunderland, MA . [ Links ]
Ungar, I.A. 1991. Ecophysiology of vascular halophytes. CRC Press, Boca Raton, FL. [ Links ]
Willadino, L. and T. Camara. 2003. Origen y naturaleza de los ambientes salinos. pp. 303-330. In: Reigosa, M.J., N. Pedrol, and A. Sánchez (eds.). La ecofisiología vegetal. International Thomson Editores Spain Paraninfo, Madrid. [ Links ]
Zekri, M. 2002. Salinity and calcium on emergence, growth and sodium and chloride concentrations of citrus rootstocks. Proc. Fla. State Hort. Soc. 106, 18-24. [ Links ]













