Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Agronomía Colombiana
versão impressa ISSN 0120-9965
Agron. colomb. vol.32 no.2 Bogotá maio/ago. 2014
https://doi.org/10.15446/agron.colomb.v32n2.42640
http://dx.doi.org/10.15446/agron.colomb.v32n2.42640
1 Research Group in Plant Ecophysiology, Faculty of Agricultural Sciences, Universidad Pedagógica y Tecnológica de Colombia (UPTC). Tunja (Colombia). fanor.casierra@uptc.edu.co
2 Faculty of Industrial Engineering, Universidad Tecnológica de Pereira (UT P). Pereira (Colombia).
Received for publication: 17 May, 2014. Accepted for publication: 30 July, 2014.
ABSTRACT
To determine the effects of light quality on the growth indices of plants, Pencas Blancas cultivar chard plantlets were grown for 2 months under five different light treatments, obtained by filtering sunlight through colored polyethylene films. The treatments included: red, blue, green, yellow and transparent cover colors. A transparent cover (white light) was used as the control. The colored covers affected the plant growth. The plants grown under the yellow cover presented a better behavior with regards to growth, taken as: total dry weight per plant, leaf area, specific leaf area, absolute growth rate, relative growth rate, harvestable dry matter and root to shoot ratio. The dry matter partitioning in the leaves and roots was affected by the light quality, but not in the petioles, with a higher accumulation of dry mass in the leaves of plants grown under the yellow cover. As a consequence of the enhanced leaf area in the plants under the yellow cover, they also had the highest water uptake. On the other hand, the highest net assimilation rate value was found in plants grown under the transparent cover. These results open up the possibility of using yellow colored cover in leafy green vegetables, especially in chard plants grown under controlled conditions.
Key words: color, dry weight, leaf area, light quality, leafiness indices, growth rates.
RESUMEN
Para evaluar el efecto de la calidad de la luz sobre los índices de crecimiento en vegetales, plántulas de acelga, cultivar Pencas Blancas, crecieron durante 2 meses expuestas a cinco tratamientos diferentes de luz obtenidos mediante el filtrado de la luz solar a través de filtros de polietileno de diferentes colores. Los tratamientos comprendieron coberturas de colores rojo, azul, verde, amarillo y transparente. La cobertura transparente (luz blanca) se tomó como control. Las coberturas coloreadas afectaron el crecimiento vegetal. Las plantas que crecieron bajo la cobertura amarilla mostraron mejor comportamiento en relación con el crecimiento, tomado como el peso seco total de las plantas, el área foliar, el área específica de las hojas, la tasa de crecimiento absoluto, la tasa de crecimiento relativo, la masa seca cosechable y la relación raíz/parte aérea. La acumulación de masa seca en hojas y raíces fue afectada por la calidad de la luz, no así en los peciolos. Se encontró mayor acumulación de masa seca en hojas de plantas que crecieron bajo la cobertura amarilla. Como consecuencia del incremento en el área foliar en plantas que estaban bajo la cobertura amarilla, estas presentaron mayor volumen de agua consumida. Por otro lado, el mayor valor de la tasa de asimilación neta se encontró en plantas colocadas bajo la cobertura transparente. Estos resultados abren la posibilidad de utilizar la cobertura amarilla en hortalizas de hoja y especialmente en plantas de acelga cultivadas bajo condiciones controladas.
Palabras clave: color, peso seco, área foliar, calidad de luz, índices de foliación, tasas de crecimiento.
Introduction
As a consequence of their sessile nature, plants need special plastics for their development; and so, show a vast range of adaptive responses to environmental cues (Whitelam, 1995). The spectral irradiance of solar radiation is a mixture of UV-radiation, photosynthetically active radiation, far red and near infrared, ranging from 280 nm to 1,100 nm (Grant, 1997). Plants sense the properties of incident light, such as its intensity, quality, direction and duration, via photoreceptors categorized as phytochromes, cryptochromes and phototropins, and use this information to regulate developmental responses, to control their structure and to determine the onset of flowering. The developmental responses of plants to the quality of the light environment are collectively referred to as photomorphogenesis (Whitelam, 1995; Lin, 2000).
Most studies have only examined a few selected light qualities (red and blue) and there are very few reports examining the effects of all the important components of light quality, such as UV, blue, green, yellow, red and far red, affecting plant growth in plants growing under what are otherwise the same environmental conditions (Li and Kubota, 2009). Macedo et al. (2011) indicated that Alternanthera plants have strong morphological plasticity induced by light. Their results suggest that high-quality Alternanthera can be achieved by growing the plants in vitro under a combination of blue and red light. Johkan et al. (2012) indicated that high-intensity green LED (light-emitting diode) light was effective in promoting plant growth and, in particular, short-wavelength green light was available for active plant growth. Lin et al. (2013) demonstrated that supplemental light quality can be strategically used to enhance the nutritional value and growth of lettuce plants grown under a combination of red, blue and white LED lights. Hultberg et al. (2014), in Chlorella vulgaris, found a higher amount of biomass produced in treatments with yellow, red and white light, in comparison with blue, green and purple light. Furthermore, in lettuce plants, fluorescent light plus red LED and fluorescent light plus blue LED resulted in the improved morphology, greater biomass and pigment contents of plants; more so than red, blue, fluorescent light or red plus blue light (Chen et al., 2014).
Chard was selected as a model crop due to its extensive distribution and use as a cultured plant. The objective of the current study was to investigate the growth responses of chard plants to light quality. Therefore, in this study, we analyzed the influence of sunlight filtered through colored filters on growth indices in chard plants using colored polyethylene plastic films.
Materials and methods
Plant material and growth conditions
The experiment was done in a greenhouse in Tunja, Colombia (5°33'10.86" N; 73°21'24.21" W - at 2,702 m a.s.l.). During the experiment, a 15.3°C average temperature and 71.4% humidity were registered in the greenhouse. Twomonth- old chard seedlings (Beta vulgaris L. var. Cicla, cv. Pencas Blancas were transferred to a nutrient solution with the following composition in mg L-1: nitric nitrogen 40.3; ammonium nitrogen 4.0; phosphorus 20.4; potassium 50.6; calcium 28.8; magnesium 11.4; sulfur 1.0; iron 1.12; manganese 0.11; copper 0.012; zinc 0.026; boron 0.106; molybdenum 1.2·10-3, and cobalt 3.6·10-4. Hypoxia around the roots was avoided by pumping air into the nutrient solution of each plant with an aquarium pump.
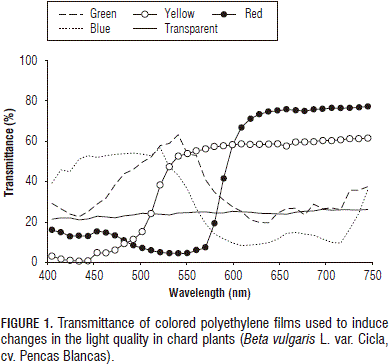
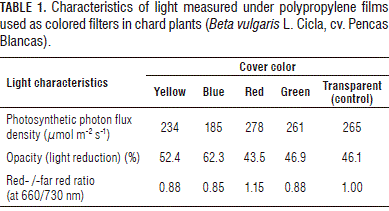
Ten plants per treatment (color) were grown under solar light filtered with 15 mm polypropylene films of the colors: red, yellow, blue, green, or transparent (white light as control). To set changes in the light quality for the treatments, colored films were put on wooden frames so that each plant grew under a structure covered by the corresponding film. Since the colored polypropylene films had different clearness levels and, in order to balance the reduction of light as much as possible, several layers of polypropylene of the same color were placed on wooden frames of the more transparent covers; in that way, the plants were mainly influenced by the color of the film, rather than by the light reduction. Photosynthetic photon flux density (PPFD), light reduction (opacity), and the ratio of red to far-red light (measured at 660±20/730±15 nm) under the different covers are shown in Tab. 1. The light /spectral transmittance of the different plastic films is presented in Fig. 1. The PPFD was determined with a Light Scout Quantum Meter 3415 (Spectrum Technologies, Inc. Plainfield, IL) and the R/FR ratio with a Field Scout Red / Far red Meter 3412 (Spectrum Technologies, Plainfield, IL).
Growth measurements
The experiment plants were harvested two months after transplanting and the dry mass (DM) of each plant organ (roots, leaves and petioles) was measured through oven drying. The leaf area was determined with a portable leaf area meter Li-Cor 3000-A (Li-Cor, Lincoln, NE). At the beginning of the experiment, the dry weight and leaf area were measured in 20 plantlets to give an initial value for the calculation of the growth indices, according to the protocols reported by Hunt (1990). The net assimilation rate (NA R) was calculated according to Gregory (1926). The water consumption (water uptake) was taken as the amount of water added weekly to the containers.
Statistical analysis
The plants were arranged in groups, using cover color as the grouping factor. Each treatment included 10 plants and each plant was taken as a replicate. The data obtained from the experiment were subjected to a classic analysis of variance and Tukey range test. All statistical analyses were performed using IBM-SPSS version 19.0.0 (IBM Corporation, Somers, NY). Results with a P value at or below (P≤0.05) were considered significant.
Results and discussion
Leafiness indices and ratios
The value of the leaf area ratio (LAR) in all the treatments with colored films exceeded the value found in the control plants grown under a transparent cover, with statistically significant differences (P≤0.01). The plants grown under the yellow cover exceeded the LAR recorded in the control plants by 128.3%, while plants grown under the red, blue and green films outperformed by a range of 55.4 to 66.2% above the LAR value of the control plants. On the other hand, all plants grown under colored covers, irrespective of the color of the cover, had a greater leaf area than plants of the control treatment (transparent cover), with significant differences (P≤0.01). The average value of leaf area registered in plants grown under yellow coverage exceeded the mean of the control plants by 361.1%. The observed increase of leaf area in plants under the blue, red and green films ranged from 80.5 and 101.8%, as compared to the control (Tab. 2). Macedo et al. (2011) recorded a smaller leaf blade area under green light in Alternanthera brasiliana plants grown in vitro and a number of leaves/plant similar to those recorded under red light, in this way it is possible to infer that phytochromes and cryptochromes are also green-light receptors, as reported by Folta and Maruhnich (2007). Therefore, it would be expected that the results for A. brasiliana under green light were similar to those under red light and blue light. Consequently, in our work, the increased leaf area shown by plants under the yellow cover was a consequence of the phytochrome activity in the plants because the yellow cover presented a maximal transmittance above 540 nm, covering a larger spectrum bandwidth, but with a maximal transmittance of red cover at 630 nm (Fig. 1).
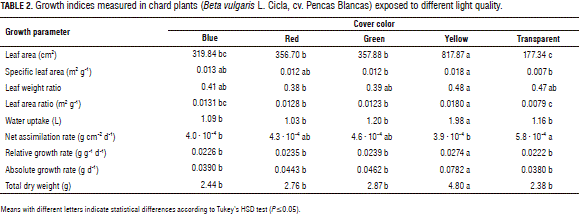
As expected, the behavior of the water uptake variable had a similar trend as leaf area, with significant differences. Plants placed under the blue and red covers showed water consumption values 5.9 and 2.6% lower than those of the control plants. Meanwhile, those plants grown under green and yellow cover presented average values of this variable in the order of 3.4 and 70.4% higher than the plants grown under the transparent cover (Tab. 2). When stomata open, water is lost to the atmosphere due to the fact that stomata can function as hydraulic valves on the surface of aerial plant parts with guard cells that surround each pore rapidly adjusting their turgor to optimize photosynthetic CO2 uptake and minimize transpiration water loss from leaves (William and Outlaw, 2003). Blue light is important in stomata opening (Kraepiel and Mipiniac, 1997); however, blue light is regulated directly by phototropins and activates a signaling cascade that results in the rapid opening of stomata under a background of red light (Shimazaki et al., 2007). On the other hand, Wang et al. (2009), while studying the stomatal density and stomatal index (number of stomata per mm2) in Cucumis sativus under different light qualities, found a lower stomatal density in plants grown under purple, yellow, green and red light than in plants grown under white light. Meanwhile, plants grown under green, yellow and red light had a lower stomatal index as compared with that of plants grown under white light. In contrast, purple-grown plants had an increased stomatal index. These studies suggest that both stomatal density and stomatal index are light quality dependent and purple and blue light also up-regulate most of the genes which encode key enzymes in the Calvin cycle, whereas green, yellow and red light down-regulate them.
Dry mass production and accumulation
Results of the biomass measurements and ratios of the chard plants influenced by the light spectra treatments are shown in Tab. 2, and Fig. 2, 3 and 4; the plants exhibited distinct growth responses to different light-quality treatments. The total DM production in the plants grown under the yellow cover increased 101.0% over the average value found in the plants under the transparent cover, with statistically significant differences. In the plants placed under the blue, red and green films, the increase in the production of dry matter in comparison to the control ranged from 2.3 to 20.4%. An increased leaf area and total DM per plant were reported by Casierra-Posada and Rojas (2009) in Brassica oleracea var. Italica plants grown under a red cover in relation to yellow, blue, red, orange and transparent cover colors. Li and Kubota (2009) found a significantly increased dry weight, stem length, leaf length and leaf width in baby leaf lettuce plants with supplemental far red light, as compared to white light, apparently due to enhanced light interception by an enlarged leaf area under supplemental far red light. A mixture of different colored light sources may combine the advantages of light quality and such a mixture may overcome the individual disadvantages of these lights. Therefore, the mixture of colored incident light with different band spectra (e.g. blue + red) promoted plantlet growth, but the best proportion of light colors may be specific to the plant species (Li et al., 2010).
Humbeck et al. (1988) reported that total irradiance is more important than spectral composition, and that, after acclimation, responses depend on the available light quantity and not quality. Indeed, photomorphogenic responses to light quality in terms of factors such as NA R are contradictory. While Boccalando et al. (2009) reported that, in Arabidopsis, a high red/far-red ratio increases stomatal index and photosynthetic rate, Xiong et al. (2011) found a positive effect on NA R when they exposed Cucumis sativus plants to far-red light. Therefore, our results showed that the yellow cover induced an evidently higher production of DM, as compared to other treatments (Tab. 2); nevertheless, the NA R in the plants under these cover colors was lower than under the transparent cover. The DM under the yellow cover presented this trend, although the light reduction under this cover color was higher (52.4%) than under the red, green and transparent films (from 43.5 to 46.9%). Even the blue film induced a light reduction of 62.3% (Tab. 1), but without statistical differences with the responses of plants grown under the red, green and transparent films in terms of total DM per plant, suggesting that phytochromes are involved in this response. Certainly, light quantity determines plant growth, according to the plasticity of each plant material, while quality of light regulates the DM production in plants together with the light quantity.
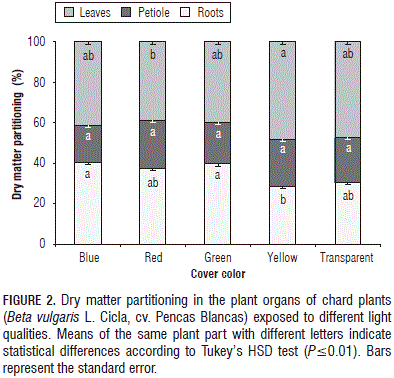
Concerning DM partitioning in the different organs, significant differences for DM accumulation in the leaves and roots were found, but not in the petioles. Compared with the control plants, under the yellow coverage, the plants reduced their DM content by 7.8% in the roots; while in those grown under the blue, red and green covers, the DM content in these organs ranged from 21.5 to 30.9% over the control. Concerning the allocation of DM in leaves, the opposite trend of the roots was found. In this case, plants grown under the yellow cover showed an increase of 1.8%, as compared with the control, whereas in plants placed under the blue, red and green films, the reduction was in the range of 12.0 to 17.3%, as compared with the control plants under the transparent cover (Fig. 2). Casierra-Posada et al. (2012a) reported that the exposure of Zantedeschia aethiopica plants to colored covers changed their pattern of DM allocation to different organs, with statistical differences in the leaves, petioles and roots, but not in the flowers. On the other hand, Casierra-Posada et al. (2011) reported the lowest percentage of DM allocation in roots of strawberry plants exposed to yellow and blue covers, while under the green cover, the plants presented the highest percentage. In this way, the architectural structure of plants is regulated, in part, by light signals from the environment. Plant characteristics controlled by photoreceptors include size, shape, height, and insertion angles of organs (Franklin et al., 2005).
The value of the specific leaf area (SLA) increased in all the plants grown under the colored films, with statistically significant differences from the control (transparent film). Under the yellow cover this increase was 128%, while under the red, blue and green covers, this increase ranged from 55.4 to 66.2% (Tab. 2), suggesting changes in the leaf thickness and the parenchyma induced by the quality of light. Macedo et al. (2011) found an increased leaf weight ratio, specific leaf mass, and leaf density in Alternanthera brasiliana plants exposed to green light. Blue light induced the largest number of leaves/plant, thickness, and leaf blade area, while red light brought the lowest specific leaf mass, thickness, and leaf density. Both red and green lights resulted in the smallest leaf areas. Both red and white light increased the dry weight/fresh weight ratio of the leaves. Garnier and Laurent (1994) found that increases in specific leaf mass correlate with increases in leaf thickness; meanwhile Macedo et al. (2011) reported that plants grown in the dark show a correlation between a higher specific leaf mass and leaf density and a lower mean leaf thickness. These results may indicate that photoassimilates were directed to increase the formation of cell structures, such as cell walls (Brown and Byrd, 1997). In addition, according to Aranda et al. (2004) and Gonçalves et al. (2008), specific leaf mass, leaf density, and leaf thickness were closely related. These authors attributed variations in leaf thickness mainly to differing proportions of mesophyll components in the leaves, according to the spectral quality used. The findings of previous studies in relation to the thickness of the leaves are contradictory, since, according to Macedo et al. (2011), blue and green light induced a greater increase of palisade parenchyma than a decrease of spongy parenchyma, but red light induced a reduction in both types of parenchyma in Alternanthera brasiliana leaves. On the other hand, Haliapas et al. (2008) found that the total leaf thickness and photosynthetic tissue volume in Petunia×hybrida plants appeared to be significantly increased by red light, as compared with white light.
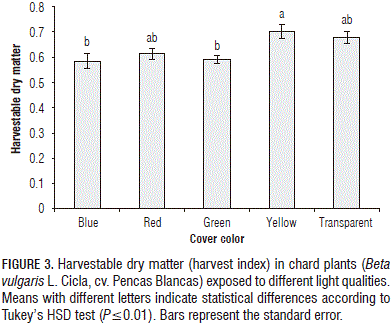
The harvestable DM (leaves+petioles/total dry mass) or harvest index rose 3.4% in plants under the yellow film color and fell in the range of 9.5 to 13.7% under the blue, red and green covers, as compared to the plants grown under the transparent cover (Fig. 3). On the other hand, the leaf weight ratio (LWR) decreased between 12.0 and 17.3% in plants placed under the red, blue and green covers, as compared with the control; however, under the yellow cover, the LWR value showed an increase of 1.8%, as compared to plants grown under the transparent film. A larger leaf area allows greater light interception, which may have led to a significant increase in biomass; a higher SLA is a good indicator of higher photosynthetic surface area per unit investment in leaf tissue (Kim et al., 2004). Johkan et al. (2010) reported that vigorous roots support shoot growth by fully supplying the plant with water and nutrients. In contrast, poor roots cannot supply sufficient water for large shoots, so plants with high shoot to root ratios are unsuitable for active growth. Nevertheless, our results showed a reduced root to shoot ratio in plants grown under the yellow cover, but, under this cover, the plants had an increased DM, leaf area and water uptake; in this way, the activity of the roots regarding water and nutrient uptake may be facilitated by means of an increased leaf area, which allowed for an increased transpiration.
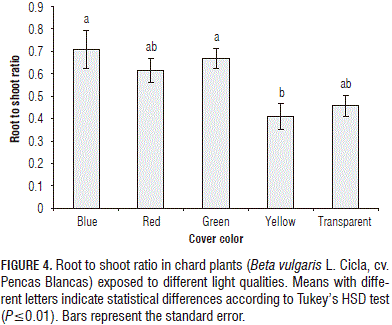
The mean of the root to shoot ratio increased in the range of 34.4 to 54.9% in plants grown under the red blue and green covers, as compared to plants placed under the transparent cover, while in plants under the yellow cover, the value of this variable was reduced by 10.3% as compared with the control (Fig. 4). In Lactuca sativa plants, Johkan et al. (2012) found that blue light, which has a short wavelength, suppressed stem elongation; whereas, red light, which has a long wavelength, promoted stem and leaf elongation. On the other hand, Nishimura et al. (2009) found enhanced leaf and stem dry weights in Perilla plants grown under red-enriched light treatments, as compared with those in other treatments; moreover, the number of true leaves and leaf area of the plants were greater in the red-enriched light treatments than those in the other treatments. These results suggest that a greater leaf area and number of true leaves increase the amount of absorbed light (photons). Therefore, the net photosynthesis rates of the plants may increase in the red-enriched light treatments, as compared with those grown in other treatments. Hence, the growth of the plants in the red enriched light treatments was promoted, as compared with those in the other treatments.
Plant growth rates
The net assimilation rate decreased in all the plants exposed to colored covers, irrespective of the film colors, as compared to plants under the transparent cover. This reduction ranged from 22.4 to 34.4%, as compared to the control (Tab. 2). The absolute growth rate (AG R) was considerably increased (105.8%) in the plants grown under the yellow cover in relation to the control; while, in plants under the blue, red and green covers, this increase was between 2.5 and 21.4% in comparison with the control plants. Furthermore, the relative growth rate (RGR), like the AG R, increased in all the plants grown under the colored covers, notably so for the plants that were under the yellow cover, with values that surpassed those of the control plants by 23.2%. On the other hand, the plants located under the red, blue and green covers presented RGR values in the range of 1.8 to 7.4% over the value of the control plants. Similarly, Casierra-Posada et al. (2012b) reported an increased NAR in strawberry plants grown under transparent cover in comparison to those under yellow, blue, green and red covers. NAR is a useful indirect measure of the photosynthetic efficiency of plants; however, the NAR values were greater in the plants grown under the transparent cover than under the yellow cover, but the total dry weight per plant showed the opposite trend. Regarding these results, Kim et al. (2004) reported that leaf CO2 assimilation rates cannot fully explain the effect on DM accumulation. These authors suggested that the cause was photosynthetic rate (Pn) measurements at a single point and that diurnal Pn and dark respiration measurements of single leaves or whole canopies would be useful to determine the fate of carbon in plants grown under lights of different qualities.
The application of supplemental lighting allows greenhouse crops to promote biomass accumulation by increasing photosynthetic carbon assimilation (Hao and Papadopoulos, 1999). Plant growth and development is strongly influenced by light quality, which refers to the color or wavelength reaching the surface of plants (Johkan et al., 2010). However, red and blue lights were reported as the light bands having the greatest impact on plant growth because they are the major energy sources for photosynthetic CO2 assimilation in photosynthetic organisms. Earlier studies examined the action spectra for photosynthesis of higher plants. It is well known that action spectra have action maxima in the blue and red ranges (Cosgrove, 1981; Kasajima et al., 2008). On the other hand, white light, combined with red and blue (red+blue+white) LED lights, was proven to be an effective lighting source for producing many plant species in controlled environments (Lin et al., 2013). Despite these reports, our results indicated a better behavior for plants grown under a yellow cover in terms of DM per plant; this cover possibly presented a maximal transmittance from 540 nm onwards (Fig. 1), covering a larger spectrum bandwidth and, in this way, several classes of photoreceptors were involved in this response.
The bands of blue and far-red light present in white light are known to have an influence on genetic expression and photomorphogenesis (Gupta et al., 2010). Kegge and Pierik (2010) reported that far-red light, despite its inefficiency for photosynthesis, gives plants relevant information on the surrounding environment. Sensors for far-red light, stimulated by red radiation and the relation between red and far-red, drive activity in molecular, biochemical, and morphological processes (Casal and Yanovsky, 2005). In this way, certain light wavelengths that are not useful for plant metabolism can influence a plant's morphology, composition, and adaptive strategy to optimize light capture when light quantity or quality is unfavorable.
Plant development is affected by the quality of light through photoreceptors known as phytocrome (which detects red and far red light), cryptochrome (blue and UV-B detector) and phototropin (blue and UV-A detector). All of these are involved in processes of photomorphogenesis, functioning independently or together to enable the plant to adapt as efficiently as possible to the environment (Spalding and Folta, 2005). All of these pigments show varying degrees of amino acid sequence identity and similarity and they induce the expression of several genes resulting in different plant responses to light quality (Briggs and Olney, 2001).
We agree with the observation of Liu et al. (2011) that, in relation to the light spectra, many reported experiments are inconsistent with light intensities that were non-uniform because the researchers were unable to precisely modulate and quantify the spectral energy parameters. Furthermore, Li and Kubota (2009) mentioned that experimental results may be partially influenced by differences in light intensity; this often presents a problem when comparing results from experiments conducted under inconsistent light parameters. While it is widely understood that light intensity can positively affect photochemical accumulation, the effects of light quality are more complex and mixed results were often reported.
Conclusions
The findings described here reveal controversial results for plant photomorphogenesis due to the fact that plants present a wide variety of photoreceptors responsible of growth and development under exposure to different light quality and, therefore, have different responses to an exposure to colored filters. Comparing our findings with other studies to obtain more conclusive approaches for the effect of light quality on plant growth is quite difficult since plants differ in their plasticity for adapting to environmental cues and, in addition, several investigations were developed using different materials. However, the positive effect of the yellow cover on the growth of the chard plants was clear in terms of the harvestable DM and leafiness indices, allowing for the possibility of using this cover color in leafy green vegetables and, especially, in chard plants grown in a greenhouse, since precise management of the irradiance and wavelength may hold promise in maximizing the economic efficiency of plant production, quality, and nutrition potential of vegetables grown under controlled environments.
Acknowledgments
This study was carried out with financing from the Research Directorate (DIN) of the Pedagogical and Technological University of Colombia (UPTC) and other support from the Plant Ecophysiological Research Group of the Agronomic Engineering Program of the Faculty of Agricultural Sciences of the same university. The authors also thank Andrés F. Vargas for assisting us in taking measurements.
Literature cited
Aranda, I., F. Pardo, L. Gil, and J.A. Pardos. 2004. Anatomical basis of the change in leaf mass per area and nitrogen investment with relative irradiance within the canopy of eight temperate tree species. Acta Oecol. 25, 187-195. [ Links ]
Boccalando, H., M.L. Rugnone, J.E. Moreno, E.L. Ploschuk, L. Serna, M.J. Yanovsky, and J.J. Casal. 2009. Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol. 150, 1083-1092. [ Links ]
Briggs, W.R. and M.A. Olney. 2001. Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 125, 85-88. [ Links ]
Brown, R.H. and G.T. Byrd. 1997. Relationships between specific leaf dry weight and mineral concentration among genotypes. Field Crops Res. 54, 19-28. [ Links ]
Casal, J.J. and M.J. Yanovsky. 2005. Regulation of gene expression by light. Int. J. Dev. Biol. 49, 501-511. [ Links ]
Casierra-Posada, F. and J.F. Rojas. 2009. Efecto de la exposición del semillero a coberturas de colores sobre el desarrollo y productividad del brócoli (Brassica oleracea var. italica). Agron. Colomb. 27, 49-55. [ Links ]
Casierra-Posada, F., J. E. Peña-Olmos, and C. Ulrichs. 2011. Crecimiento y eficiencia fotoquímica del fotosistema II en plantas de fresa (Fragaria sp.) afectadas por la calidad de la luz: Implicaciones agronómicas. Rev. UDCA Act. & Div. Cient. 14, 43-53. [ Links ]
Casierra-Posada, F., P. J. Nieto. and C. Ulrichs. 2012a. Crecimiento, producción y calidad de flores en calas (Zantedeschia aethiopica (L.) K. Spreng) expuestas a diferente calidad de luz. Rev. UDCA Act. & Div. Cient. 15, 97-105. [ Links ]
Casierra-Posada, F., J.E. Peña-Olmos, and C. Ulrichs. 2012b. Basic growth analysis in strawberry plants (Fragaria sp.) exposed to different radiation environments. Agron. Colomb. 30, 25-33. [ Links ]
Chen, X.L. W.Z. Guo, X.Z. Xue, L.C. Wang, and X.J. Qiao. 2014. Growth and quality responses of 'Green Oak Leaf' lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 172, 168-175. [ Links ]
Cosgrove, D. 1981. Rapid suppression of growth by blue light. Plant Physiol. 67, 584-590. [ Links ]
Folta, K.M. and S.A. Maruhnich. 2007. Green light: a signal to slow down or stop. J. Exp. Bot. 58, 3099-3111. [ Links ]
Franklin, K.A., V.S. Larner, and G.C. Whitelam. 2005. The signal transducing photoreceptors of plants. Int. J. Dev. Biol. 49, 653-664. [ Links ]
Garnier, E. and G. Laurent. 1994. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol. 128, 725-736. [ Links ]
Gonçalves, B., C.M. Correia, A.P. Silva, E.A. Bacelar, A. Santos, and J.M. Moutinho-Pereira. 2008. Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci. Hortic. 116, 381-387. [ Links ]
Grant, R.H. 1997. Partitioning of biologically active radiation in plant canopies. Int. J. Biometeorol. 40, 26.40. [ Links ]
Gregory, F.G. 1926. The effect of climatic conditions on the growth of barley. Ann. Bot. 40, 1-26. [ Links ]
Gupta, V., A. Roy, and B.C. Tripathy. 2010. Signaling events leading to red-light-induced suppression of photomorphogenesis in wheat (Triticum aestivum). Plant Cell Physiol. 51, 1788-1799. [ Links ]
Haliapas, S., T.A. Yupsanis, T.D. Syros, G. Kofidis, and A.S. Economou. 2008. Petunia × hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol. Plant 30, 807-815. [ Links ]
Hao, X. and A.P. Papadopoulos. 1999. Effects of supplemental lighting and cover materials on growth, photosynthesis, biomass partitioning, early yield and quality of greenhouse cucumber. Sci. Hortic. 80, 1-18. [ Links ]
Hultberg, M., H. Larsson, K. J. Bergstrand, and A. S. Carlsson. 2014. Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour. Technol. 159, 465-467. [ Links ]
Humbeck, K., B. Hoffman, and H. Senger. 1988. Influence of energy flux and quality of light on the molecular organization of the phytoplankton apparatus in Scenedesmus. Planta173, 205-212. [ Links ]
Hunt, R. 1990. Basic growth analysis. Plant growth analysis for beginners. Unwin Hyman, Boston, MA. [ Links ]
Johkan, M., K. Shoji, F. Goto, S. Hashida, and T. Yoshihara. 2010. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 45, 1809-1814. [ Links ]
Johkan, M., K. Shoji, F. Goto, S. Hahida, and T. Yoshihara. 2012. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 75, 128-133. [ Links ]
Kasajima, S., N. Inoue, R. Mahmud, and M. Kato. 2008. Developmental responses of wheat cv. Norin 61 to fluence rate of green light. Plant Prod. Sci. 11, 76-81. [ Links ]
Kegge, W. and R. Pierik. 2010. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 15, 126-132. [ Links ]
Kim, H.H., G.D. Goins, R.M. Wheeler, and J.C. Sager. 2004. Greenlight supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 39, 1617-1622. [ Links ]
Kraepiel, Y. and E. Mipiniac. 1997. Photomorphogenesis and phytohormones. Plant Cell Environ. 20, 807-812. [ Links ]
Li, Q. and C. Kubota. 2009. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 67, 59-64. [ Links ]
Li, H.M., Z.G. Xu, and C.M. Tang. 2010. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tiss. Organ Cult. 103, 155-163. [ Links ]
Lin, C. 2000. Photoreceptors and regulation of flowering time. Plant Physiol. 123, 39-50. [ Links ]
Lin, K.-H., M.Y. Huang, W.D. Huang, M.H. Hsu, Z.W. Yang, and C.M. Yang. 2013. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 150, 86-91. [ Links ]
Liu, M., Z. Xu, and Y. Yang. 2011. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tiss. Organ Cult. 106, 1-10. [ Links ]
Macedo, A.F., M.V. Leal-Costa, E.S. Tavares, C.L.S. Lage, and M.A. Esquibel. 2011. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 70, 43-50. [ Links ]
Nishimura, T., K. Ohyama, E. Goto, and N. Inagaki. 2009. Concentrations of perillaldehyde, limonene, and anthocyanin of Perilla plants as affected by light quality under controlled environments. Sci. Hortic. 122, 134-137. [ Links ]
Shimazaki, K., M. Doy, S.M. Assmann, and T. Kinoshita. 2007. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58, 219-247. [ Links ]
Spalding, E.P. and K.M. Folta. 2005. Illuminating topics in plant photobiology. Plant Cell Environ. 28, 39-53. [ Links ]
Wang, H., M. Gu, J. Cui, K. Shi, Y. Zhou, and J. Yu. 2009. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B. 96, 30-37. [ Links ]
Whitelam, G. 1995. A green light for cryptochrome research. Curr. Biol. 5, 1351-1353. [ Links ]
William, H. and Jr. Outlaw. 2003. Integration of cellular and physiological functions of guard cells. Crit. Rev. Plant Sci. 22, 503-529. [ Links ]
Xiong, J., G.G. Patil, R. Moe, and S. Torre. 2011. Effects of diurnal temperature alternations and light quality on growth, morphogenesis and carbohydrate content of Cucumis sativus L. Sci. Hortic. 128, 54-60. [ Links ]













