Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.32 no.3 Bogotá Sept/Dec. 2014
https://doi.org/10.15446/agron.colomb.v32n3.46809
Doi: 10.15446/agron.colomb.v32n3.46809
1 Biotechnology Group, Biology Institute, Universidad de Antioquia. Medellin (Colombia). estherjulia@gmail.com
2 Group Coordinator.
Received for publication: 26 September, 2014. Accepted for publication: 27 November, 2014.
ABSTRACT
Anthurium antioquiense Engl. is a native plant belonging to the Araceae family. It grows on rocks in clear-water rivers and well-protected zones, similar to the waters in certain watersheds of the Antioquia Department, Colombia. Loss of habitat has threatened this promising ornamental plant species, which is also important because of its role in the ecosystem. In vitro tissue culture is considered an efficient alternative for the propagation of endangered species with the aim of establishing short-, medium- and long-term conservation programs. In the present research, in vitro introduction and shoot induction from A. antioquiense seedlings were performed. The highest production of shoots was obtained in a ½ MS (half-salt content) medium with 1 mg L-1 of BAP, which attained a 23.7 shoots/explant per month multiplication rate. The in vitro plants generated from shoots were individualized and transferred to a growth regulator-free medium. Rooting did not require the presence of growth regulators, and the adaptation of the in vitro plants to ex vitro conditions achieved a 98% survival rate.
Key words: Anthurium, growth regulators, in vitro culture, preservation, propagation, shoots.
RESUMEN
Anthurium antioquiense Engl. es una planta nativa perteneciente a la familia Araceae. Crece sobre rocas en ríos de aguas claras y en zonas protegidas, características de algunas cuencas hidrográficas del departamento de Antioquia-Colombia. La pérdida de hábitat amenaza esta promisoria especie ornamental, la cual también es importante debido a su incidencia en el ecosistema. El cultivo de tejidos in vitro se considera una alternativa eficiente para la propagación de las especies en peligro de extinción, con el fin de establecer programas de conservación a corto, mediano o largo plazo. En el presente trabajo de investigación, se llevó a cabo la introducción e inducción de brotes in vitro de A. antioquiense. La mayor producción de brotes se obtuvo en medio ½ MS (contenido de sales a la mitad) con 1 mg L-1 de BAP (23,7 brotes/explante por mes). Las plantas in vitro obtenidas a partir de los brotes fueron individualizadas y transferidas a medio libre de reguladores de crecimiento. El enraizamiento se realizó sin presencia de reguladores de crecimiento, y la adaptación de las plantas in vitro a condiciones ex vitro alcanzó una tasa de supervivencia del 98%.
Palabras clave: Anthurium, reguladores de crecimiento, cultivo in vitro, preservacion, propagacion, brotes.
Introduction
A large number of endemic and native species are located in a variety of areas of the planet, with particular characteristics (hotspots); many of them have suffered a significant loss of habitat (Malcolm et al., 2006). The biodiversity hotspots consist of 35 biogeographic regions that cover 17.3% of the Earth's estimated land surface and contain 50% of the world's plant species (Myers et al., 2000; Mittermeier et al., 2011); of these, 10 largely tropical biogeographical regions have exceptional biodiversity and habitat destruction (Sloan et al., 2014).
Colombia is the second richest country in terms of species in the world and it has the richest collection of flora with about 45,000 species of vascular plants (Sofrony, 2007). According to the IUCN (International Union for Conservation of Nature) and information obtained from the Red Books of endangered species, of 1,476 evaluated species, 31% (458 species) are threatened, which suggests that nearly 30% of the Colombian flora may be threatened as well (García, 2007). Conservation measures must be taken to ensure the sustainable use of species that have a potential value (alimentary, medical, ornamental, etc.), are of ecological importance or are endangered.
Anthurium antioquiense Engl. is popularly known as "anturio antioquense" in Colombia. It grows in clear-water rivers and protected areas; it is an epipetric or terrestrial plant with simple leaves that are alternated and closely elliptical with a complete margin, accuminate apex and base, and grows to a height of 0.3-0.5 m. Spadix white, spathe pink-violet in color, it grows abundant fruits, green- to white-colored berries. It is native to Colombia and has a reduced distribution in natural populations. A. antioquiense is described as primarily occurring in the Antioquia department, where the type collection is located (Cardona et al., 2011; Tropicos.org, 2014). It has been found in pre-mountainous and tropical wet forests at an altitude of 500-2,000 m a.s.l. (Idárraga and Callejas, 2011). Its potential use is as an ornamental flower for interior decoration, in flower pots and floral arrangements; it is appreciated for its size and ease of management.
Currently, there is little or no published information on A. antioquiense for important aspects of the species, such as its phenology, propagation systems (ex situ, in situ and in vitro), or seed viability, etc. Wang et al. (1998) evaluated the resistance or tolerance to nematodes in different species of anthurium, including A. antioquiense. In this study, the plant material experienced in vitro handling (non-detailed protocol), and seeds were used; the authors mention the species distribution, referencing Colombia. A. antioquiense has been included in other studies of the family (Araceae) and genus (Anthurium), in which the number of chromosomes and pollen type were determined (Sheffer and Croat, 1983; Weber et al., 1999). Kuanprasert et al. (1998) and Venkat et al. (2014) mentioned this anthurium plant in producing hybrids by crossing it with commercial species such as A. andreanum. None of the above papers gave more information about this species.
Species of the genus Anthurium are usually propagated by seeds, which have a low propagation rate and low viability; they cannot be stored and the time of plant development to production of mature seeds is approximately 3 years; making the development of breeding and improvement programs difficult (Dufour and Guérin, 2006). A. andreanum is the most studied species in the genus, it has several micropropagation protocols that include organogenesis and embryogenesis, with a differential response in a variety of media and culture conditions (Joseph et al., 2003; Vargas et al., 2004; Te-Chato et al., 2006; Viégaset al., 2007; Atak and Çelik, 2009; Liendo and Mogollón, 2009; Yu et al., 2009; Islam et al., 2010; Oropeza et al., 2010; Atak and Çelik, 2012; Farsi et al., 2012).
Biotechnology provides opportunities for the propagation and conservation of species of endangered plants and/or plants of commercial interest. The ornamental plant industry has applied plant tissue culture techniques for the large-scale production of elite material (Rout et al., 2006) and for establishing tissue banks as a method of preserving genetic diversity when seeds cannot be stored in germoplasm banks (Pence, 2011). For conservation purposes, a collection of plant cuttings and seeds is the most used method. In order to preserve the maximum diversity (genetic variability), the accessible seeds of endangered species are commonly preferred; many laboratories have used in vitro methods of seed germination to conserve numerous rare plants, such as cacti, lilies and some orchid species. The seeds produced by endangered species are mostly very small in number or they do not have the potential to germinate; in vitro germination is frequently used to germinate disease-free seedlings when there are few seeds available; these seedlings are then used tips of shoot and nodes and act as explants for the propagation (Maryam et al., 2014).
The purpose of this study was to obtain a simple in vitro propagation protocol for the native and endangered species A. antioquiense for its conservation and future sustainable use.
Materials and methods
The plant material and source of the explants were collected in San Luis, La Cristalina district, Antioquia, Colombia. The collected plants were planted at the biological station of the Biology Institute, Nature and Exact Sciences Faculty, Universidad de Antioquia (Fig. 1A). The plant material was kept in pots filled with the substrate from the collection site mixed with bark from Pinuspatula, under screenhouse conditions. The experiment was conducted at the Plant Tissues Culture Laboratory, Plant Biotechnology Group (BioVeg), Biology Institute, Natural and Exact Sciences Faculty, University of Antioquia, Colombia.

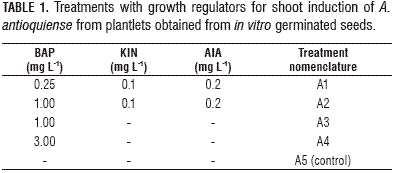
Seedlings with 2-3 leaves were used for shoot induction, which were obtained in vitro from the MS culture medium (Murashige and Skoog, 1962) at a half salts concentration (½ MS), supplemented with 20.0 g L-1 sucrose, 20.0 mg L-1 adenine and 2.6 g L-1 phytagel. For shoot induction, the same basal medium was used with the addition of the growth regulator 6-benzylaminopurine (BAP), alone or in combination with kinetin (KIN) and indole-3-acetic acid (AIA); the basal medium without growth regulators was used as the control treatment (Tab. 1). The cultures were kept at 23±2°C under lighted conditions. The medium pH was adjusted to 5.75 with NaOH or HCl 1 N solutions. The culture media were sterilized at 121°C and 15 psi (103,42 kPa) for 20 min. The number of shoots was observed through a stereoscope (Olympus SZ40, Tokyo, Japan) and counted at 60 dai.
The shoots developed in the different treatments were transferred after 60 d to vessels containing the above growth regulator-free culture medium. The material was maintained at 23±2°C under constant light, and the number of developed and rooted plants by treatment was counted.
For the adaptation process, 220 plantlets were selected (1.5-3.0 cm). The in vitro plants were transplanted to a tray with sterile soil (garden soil or black soil), maintained in covered and with semi-controlled conditions of light and temperature. The presence of fungi was controlled with Benomil® (500 mg L-1) sprayings, and, after 60 d, the in vitro plants were totally exposed to environmental conditions and hydrated once per week.
For the statistical analysis, a completely random factorial experiment was performed with four repetitions per treatment and four explants per repetition. The obtained data were analyzed with the Statgraphics® Centurion XVI software (StatPoint Technologies, Warrenton, VA); an analysis of variance (ANOVA) and a Fisher's Least Significant Difference (LSD) analysis were performed.
Results
Over 90% of the seeds germinated in the MS½ culture medium under constant light. Germination started at 3 d, and after 15 d, complete seedlings were obtained (Fig. 1B-G).
For the shoot induction process, the analysis of variance (ANOVA) showed a statistically significant difference (P≤0.05) between the treatments, with a confidence level of 95%. The combination of evaluated growth regulators had an effect on the shoot formation. The Fisher's LSD interval analysis classified the treatments into three homogeneous groups. Treatments A1-A4 showed statistically significant differences compared to the control treatment (A5). The treatments exhibited significant differences between A4 and A3 and between A1 and A3. The A2 treatment did not show a statistically significant difference with treatment A3 (Tab. 2, Fig. 2).
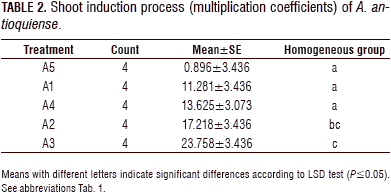
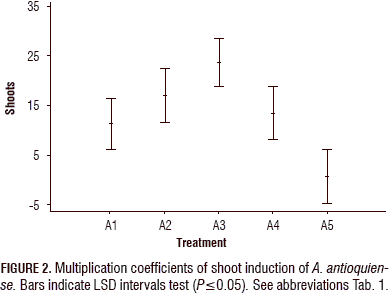
The highest multiplication coefficient (23.25 shoots/explant per month) was obtained in treatment A3, containing BAP at 1 mg L-1; followed by the treatment A2, containing BAP (1.0 mg L-1) in combination with KIN (0.1 mg L-1) and AIA (0.2 mg L-1) (17.2 shoots/explant per month). In the A4 treatment, a more compact structure was formed; in the control (A5), the multiplication coefficient was the lowest (1.79 shoots/explant per month) (Figs. 2 and 3).

The rooting process began spontaneously without an addition of growth regulators, even before the individualization (Fig. 4). The individualization and transference to this medium allowed for the improvement of the growth and development of the plantlets and roots; the latter appeared in 98% of the in vitro plants developed from shoots. The rooting lasted between 20 and 40 d, and most of the plantlets presented 3-6 roots; 4 months after individualization all of the plants presented roots.

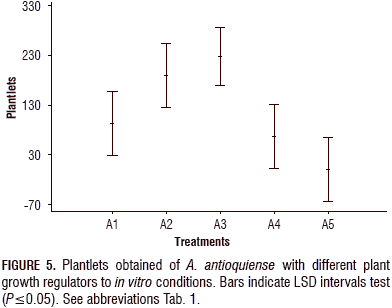
The analysis of variance ANOVA and significant difference (LSD) analysis were also performed for the number of in vitro plants developed in each treatment and for the number of plants that formed roots. Similar to the shoot induction, the analysis showed significant differences between the treatments with growth regulators and the control treatment, which is consistent with the results shown in the tables and graphs for shoot induction. The highest number of developed plants and plants with roots was obtained with treatment A3, and the lowest number was obtained in the control treatment (Fig. 5).
The adaptation to soil conditions was successful, achieving 98.2% (216 plants) well-adapted seedlings; there were no visible differences between seedlings of 1.5 and 3.0 cm in terms of adaptation response. The plants showed elongation of the leaves, typical of plants that develop in natural conditions (Fig. 6).

Discussion
The germination of this native anthurium was relatively easy and quick in the laboratory (in vitro), in contrast to direct soil germination (observed in preliminary tests, data not shown), which was not successful, possibly because the culture medium supplied the nutrients needed for this process and because the conditions were controlled; there was no pathogen or agent that attacked the germinating seedlings.
The A. antioquiense germination rate (>90%) found in the present study was optimal and exceeded the rate obtained for A. andreanum by Vargas et al. (2004), who obtained a 74% germination rate in a MS medium compared to a supplemented medium with BAP, which only produced a 30% germination rate. Conversely, Oropeza et al. (2010) found that a MS medium with 0.5 mg BAP was suitable for the in vitro germination of seeds and for establishing micro-cuttings of A. andreanum Lind cv. Rubrun. For the Anthurium genus, several studies have used complete and half concentrations of basal medium MS for seed germination as well as for regeneration and development of shoots and rooting, obtaining good results (Joseph et al., 2003; Martin et al., 2003; Viégas et al., 2007; Yu et al., 2009). Te-chato et al. (2006) evaluated the response to an in vitro culture of several Anthurium genotypes in different basal media and found that the best response was obtained in a MS medium.
The two better shoot multiplication rates obtained in the ½ MS culture medium contained 1 mg L-1 of BAP (A3 and A2 treatments) (Figs. 2, 3); this phytohormone has been reported in anthuriums by various authors. Leffringen and Soede (1979) were the first to describe the use of BAP as the only growth regulator to induce shoots in Anthurium; they used a concentration of 1-3 mg L-1. Shoot induction using BAP at 1 mg L-1 has also been reported for this genus by Pierik (1976). Liendo and Mogollón (2009) obtained similar results as the present study for A. andreanum; they produced a larger number of shoots in a medium with 1 mg L-1 of BAP; additionally, the use of the same medium with different concentrations of the growth regulator naphthaleneacetic acid (NAA) reduced the number of shoots, but a 0.01 mg L-1 concentration of NAA positively influenced the shoot length. Islam et al. (2010) indicated that the addition of NAA at variable concentrations in a medium that contained 1 mg L-1 BAP produced shoot induction in A. andreanum cv. Nitta.
The formation of compact masses in A4 was apparently composed of shoots that could not develop and may have been the result of the 3 mg L-1 BAP content in the culture medium (Fig. 3), which might suggest that this is high for this species. Yu et al. (2009) used the growth regulator combination of 2,4-D (2,4-Dichlorophenoxyacetic acid) and BAP at different concentrations; they reported the formation of compact masses that corresponded to the asynchronous development of a callus mass from explants of the leaf and petiole in A. andreanum; the authors described the mass as the formation of structures similar to protocorm, from which the plant formations were indirectly obtained.
The development of the shoots from the initial seedlings continued in the growth regulator-free culture medium for 2-3 months after the data registry, which suggested that the addition of BAP in the induction phase was enough to stimulate the shoot formation process in many consecutive sub-cultures. This result should be evaluated to optimize the use of cytokinin and prevent possible negative effects on A. antioquiense plants propagated through this method.
Rooting for this anthurium does not require the use of phytohormones; the occurrence of spontaneous roots has already been reported by other authors for this genus (Viégas et al., 2007; Liendo and Mogollón, 2009); therefore, one specific medium for forming this organ is not necessary. Recently, Farsi et al. (2012) also reported rooting without the addition of phytohormones for the micropropagation of A. Andreanum cv. Terra. Furthermore, different researchers have reported rooting in 84 to 100% of the shoots of different varieties of A. andreanum using growth regulators (Martin et al., 2003; Bejoy et al., 2008; Islam et al., 2010).
The results of the ex vitro adaptation revealed the relative easy acclimation of A. antioquiense plantlets to environmental conditions, using garden soil and controlling the initial conditions and appearance of fungi (Fig. 6). After the plants are established, great care is not required, except to water them. Some authors have reported different substrates for in vitro regenerated acclimatization (Martin et al., 2003; Viégas et al., 2007); vermicompost and sand mixture (1:3 relation), vermiculite and perlite (1:1), and soil and organic humus (1:1) are the more common acclimatization media, with high survival ratios ranging from 60% to 98%(Atak and Çelik, 2012).
At the end of this study, 2,300 plantlets (in vitro) were obtained from all of the evaluated treatments in which they shooted.
Conclusions
This is the first report of a complete protocol of in vitro multiplication for A. antioquiense as a starting point for future conservation and sustainable use programs for this promising species and for use as a model for other related and endangered species. This protocol was efficient and reproducible (obtaining plants adapted to soil and environmental conditions), in which induced shoots from in vitro seedlings were obtained using the growth regulator BAP (1 mg L-1) in a basal ½ MS medium, in which the best multiplication rate (23.7 shoots/explant per month) was seen. The rooting process, occurring spontaneously, does not require the addition of phytohormones; the good production of roots facilitates the adaptation of plantlets to ex vitro conditions, which is optimal under controlled conditions. The in vitro propagation of anthuriums from seeds is a quick and reliable way to get a high amount of seedlings in a small amount of space at any time of year that are free of pathogens; the obtained seedlings can be gradually adapted to soil until complete and successfully acclimatization for the proper development, maturation and production of new seeds.
Recommendations
The initial control of fungal growth is strongly recommended for good adaptation of plantlets to soil during the acclimation process using a commercial fungicide. After establishment, no further use is needed.
Despite a good germination percentage obtained in vitro, an evaluation of substrates other than black soil is recommended, such as moss, for the germination of seeds obtained directly from plants, along with a comparison of in vitro and ex vitro germination.
A population study is required to determine the current conditions and distribution of native anthurium in Colombia because of the sensitive nature of its habitat and the difficulty of finding natural populations during the search for plant material in the present study at reported sites in the department of Antioquia.
Acknowledgments
The authors express their gratitude to the Comitépara el Desarrollo de la Investigación (CODI) for researching (2011) and providing a funding source for this project; to the Biotechnology group and all members of the Plant Tissue Culture Laboratory (BioVeg) of the Universidad de Antioquia for providing space and infrastructure and making the development of this project possible; to Herber Sarrazola for his collaboration on the statistical analyses; and to the Universidad de Antioquia, CODI Sustainability Strategy 2013-2014, for the support given to the Biotechnology group.
Literature cited
Atak, Ç. and Ö. Çelik. 2009. Micropropagation of Anthurium andraeanum from leaf explants. Pak. J. Bot. 41, 1155-1161. [ Links ]
Atak, Ç. and Ö. Çelik. 2012. Micropropagation of Anthurium spp. pp. 241-254. In: Dhal, N.K. and S.C. Sahu (eds.). Plant science. InTech Press, Rijeka, Croatia. Doi: 10.5772/51426 [ Links ]
Bejoy, M., V.R. Sumitha, and N.P. Anish. 2008. Foliar regeneration in Anthurium andraeanum Hort. cv. Agnihothri. Biotechnology 7, 134-138. Doi: 10.3923/biotech.2008.134.138 [ Links ]
Cardona, N.F., D. Higuita, S. Gómez, and F. Roldán. 2011. Flora de embalses. Illustrated guide. ISAGEN; Herbario Universidad de Antioquia, Medellin, Colombia. [ Links ]
Dufour, L. and V. Guérin. 2006. Main environmental factors affecting flowering of Anthurium andreanum L. soilless cultivated in tropical conditions. pp. 172-182. In: Teixeira, J.A. (ed.). Floriculture, ornamental and plant biotechnology: advances and topical issues. Vol. 3. Global Science Books, Takamatsu, Japan. [ Links ]
Farsi, M., M.E. Taghavizadeh Y., and V. Qasemiomran. 2012. Micropropagation of Anthurium andreanum cv. Terra. Afr. J. Biotechnol. 11, 13162-13166. Doi: 10.5897/AJB12.893 [ Links ]
García, N. 2007. Libro rojo de plantas de Colombia. Vol. 5: Las magnoliáceas, las miristicáceas y las podocarpáceas. Serie Libros Rojos de Especies Amenazadas de Colombia. Instituto Alexander von Humboldt; Corantioquia; Jardín Botánico Joaquin Antonio Uribe de Medellín; Instituto de Ciencias Naturales de la Universidad Nacional de Colombia; Ministerio de Ambiente Vivienda y Desarrollo Territorial, Bogota. [ Links ]
Idárraga, P.A. and P.R. Callejas. 2011. Análisis florístico de la vegetación del departamento de Antioquia. p. 271. In: Idárraga P., A., R.C. Ortiz, R. Callejas, and M. Merello (eds.). Flora de Antioquia: Catálogo de las plantas vasculares. Vol. 2. Series Biodiversidad y Recursos Naturales. Universidad de Antioquia; Missouri Botanical Garden; Oficina de Planeación Departamental de la Gobernación de Antioquia, Bogota. [ Links ]
Islam, S.A., M.M.R. Dewan, M.H.R. Mukul, M.A. Hossain, and F. Khatun. 2010. In vitro regeneration of Anthurium andreanum cv. Nitta. Bangladesh J. Agril. Res. 35, 217-226. Doi: 10.3329/bjar.v35i2.5884 [ Links ]
Joseph, D., K.P. Martin, J. Madassery, and V.J. Philip. 2003. In vitro propagation of three commercial cut flower cultivars of Anthurium andraeanum Hort. Indian J. Exp. Biol. 41, 154-159. [ Links ]
Kuanprasert, N., A.R. Kuehnle, and C.S. Tang. 1998. Floral fragrance compounds of some Anthurium (Araceae) species and hybrids. Phytochemistry 49, 521-528. Doi: 10.1016/S0031-9422(98)00088-0 [ Links ]
Leffringen, I.L. and A.C. Soede. 1979. Weefselkweek Anthurium andraeanum onderzoek te boven (2). Vakblad. Bloem. 34, 40-41. [ Links ]
Liendo, M. and N. Mogollón. 2009. Multiplicación clonal in vitro del anturio (Anthurium andreanum Lind. cv. Nicoya). Bioagro 21, 179-182. [ Links ]
Malcolm, J.R., C. Liu, R.P. Neilson, L. Hansen, and L. Hannah. 2006. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 20, 538-548. Doi: 10.1111/j.1523-1739.2006.00364.x [ Links ]
Martin, K.P., D. Joseph, J. Madasser, and V.J. Philip. 2003. Direct shoot regeneration from lamina explants of two commercial cut flower cultivars of Anthurium andraeanum Hort. In Vitro Cell. Dev. Biol. Plant 39, 500-504. Doi: 10.1079/IVP2003460 [ Links ]
Maryam, A., R. Tariq, S. Chuadhary, R. Azmat, S. Javed, and S. Khanam. 2014. A review: Role of tissue culture (in-vitro) techniques in the conservation of rare and endangered species. Pac. J. life Sci. 2, 93-103. [ Links ]
Mittermeier, R.A., W.R. Turner, F.W. Larsen, T.M. Brooks, and C. Gascon. 2011. Global biodiversity conservation: the critical role of hotspots. pp. 3-22. In: Zachos, F.E. and J.C. Habel (eds.). Biodiversity hotspots: Distribution and protection of priority conservation areas. Springer-Verlag, Berlin. Doi: 10.1007/978-3-642-20992-5_1 [ Links ]
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473-497. Doi: 10.1111/j.1399-3054.1962.tb08052.x [ Links ]
Myers, N., R.A. Mittermeier, C.G. Mittermeier, G.A.B. Fonseca, and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853-858. Doi: 10.1038/35002501 [ Links ]
Oropeza, M., M. Alexander, and T.E. Vargas. 2010. Micropropagation and organogenesis of Anthurium andreanum Lind. cv. 'Rubrun'. Methods Mol. Biol. 589, 3-14. Doi: 10.1007/978-1-60327-114-1_1 [ Links ]
Pence, V.C. 2011. Evaluating costs of the in vitro propagation and preservation of endangered plants.In Vitro Cell. Dev. Biol. Plant 47, 176-187. Doi: 10.1007/s11627-010-9323-6 [ Links ]
Pierik, R.L.M. 1976. Anthurium andraeanum plantlets produced from callus tissues cultivated in vitro. Plant Physiol. 37, 80-82. Doi: 10.1111/j.1399-3054.1976.tb01876.x [ Links ]
Rout, G.R., A. Mohapatra, and S. Mohan Jain. 2006. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 24, 531-560. Doi: 10.1016/j.biotechadv.2006.05.001 [ Links ]
Sheffer, R.D. and T.B. Croat. 1983. Chromosome numbers in the genus Anthurium (Araceae) II. Amer. J. Bot. 70, 858-871. Doi: 10.2307/2442938 [ Links ]
Sloan, S., C.N. Jenkins, L.N. Joppa, D.L.A. Gaveau, and W.F. Laurance. 2014. Remaining natural vegetation in the global biodiversity hotspots. Biol. Conserv. 177, 12-24. Doi: 10.1016/j.biocon.2014.05.027 [ Links ]
Sofrony, E. 2007. Rescue program for endangered species in Colombia. Actual. Biol. 9, 37. [ Links ]
Te-Chato, S., T. Susanon, and Y. Sontikun. 2006. Cultivar, explant type and culture medium influencing embryogenesis and organogenesis in Anthurium spp. Songklanakarin J. Sci. Technol. 28, 717-722. [ Links ]
Tropicos.org. 2014. Plants database. Missouri Botanical Garden, Saint Louis, MO. In: http://www.tropicos.org; consulted: November, 2014. [ Links ]
Vargas, T.E., A. Mejías, M. Oropeza, and E. García. 2004. Plant regeneration of Anthurium andreanum cv. Rubrun. Electron. J. Biotechn.7, 282-286. Doi: http://dx.doi.org/10.2225/vol7-issue3-fulltext-11 [ Links ]
Venkat, S.K., P. Bommisetty, M.S. Patil, L. Reddya, and A. Chennareddy. 2014. The genetic linkage maps of Anthurium species based on RAPD, ISSR and SRAP markers. Sci. Hortic. 178, 132-137. Doi: 10.1016/j.scienta.2014.08.017 [ Links ]
Viégas, J., M.T.R. Rocha, I. Ferreira-Moura, D.L. Rosa, J.A. Souza, M.G.S. Correa, and J.A.T. Silva. 2007. Anthurium andraeanum (Linden ex André) culture: in vitro and ex vitro. Floric. Ornam. Biotech. 1, 61-65. [ Links ]
Wang, K.H., A.R. Kuehnle, and B.S. Sipes. 1998. In vitro tolerance and resistance to burrowing nematode, Radopholus similis, in Anthurium species. Euphytica 103, 23-28. Doi: 10.1023/A:1018384019938 [ Links ]
Weber, M., H. Halbritter, and M. Hesse. 1999. The basic pollen wall types in Araceae. Int. J. Plant Sci. 160, 415-423. Doi: 10.1086/314122 [ Links ]
Yu, Y.X., L. Liu, J.X. Liu, and J. Wang. 2009. Plant regeneration by callus-mediated protocorm-like body induction of Anthurium andraeanum. Hort. Agric. Sci. China 8, 572-577. Doi: 10.1016/S1671-2927(08)60248-5 [ Links ]













