Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.33 no.1 Bogotá Jan./Apr. 2015
https://doi.org/10.15446/agron.colomb.v33n1.48241
Doi: 10.15446/agron.colomb.v33n1.48241
1 School of Chemistry, Politécnico Colombiano Jaime Isaza Cadavid. Medellin (Colombia)
2 Plant Health and Biological Control Unit, Corporación para Investigaciones Biológicas. Medellin (Colombia). davidgranada888@gmail.com
3 Faculty of Health Sciences, Institución Universitaria Colegio Mayor de Antioquia. Medellin (Colombia)
4 Faculty of Sciences, Universidad del Tolima. Ibague (Colombia)
Received for publication: 14 January, 2015. Accepted for publication: 30 March, 2015.
ABSTRACT
The demand for Hass avocado in the global market exceeds the supply by over 50%. Colombia has a remarkable advantage as a producer in the region due to its high yields. However, the productivity of this crop can be seriously affected by diseases such as root rot, caused by Phytophthora cinnamomi, postharvest body rot and stem end rot, caused by Colletotrichum sp. and Phomopsis sp., respectively. The potential of 76 bacterial isolates obtained from avocado rhizosphere to produce inhibitory metabolites against avocado's pathogens was evaluated. The antagonistic effect of the rhizobacteria against P. cinnamomi, Colletotrichum sp. and Phomopsis sp. was tested through dual cultures. Thirty-six percent of the tested isolates presented inhibition halos against P. cinnamomi, 36% against Colletotrichum sp. and 67% against Phomopsis sp. Additionally, three isolates were selected for fermentation tests using different broth cultures. The extracts obtained from fermentations in the minimal medium of isolates ARP5.1 and AED06 showed inhibitory activity against the evaluated pathogens, but this effect was not observed with the AED26 extract. The media supplemented with copper chloride did not enhance activity of the extracts. These results suggest that using microbial metabolic extracts is a viable alternative for controlling avocado pathogens in vitro.
Key words: Persea americana, postharvest diseases, Phytophthora cinnamomi, secondary metabolites, rhizobacteria.
RESUMEN
La demanda mundial de aguacate Hass supera la oferta en más de un 50%. A pesar de los altos rendimientos de este cultivo en Colombia, la productividad se ve seriamente afectada por enfermedades como la pudrición de la raíz, causada por Phytophthora cinnamomi, y las pudriciones poscosecha causadas por Colletotrichum sp. y Phomopsis sp. En este trabajo se evaluó el potencial de 76 aislamientos bacterianos obtenidos de la rizósfera de plantas de aguacate, para la producción de metabolitos con actividad inhibitoria frente a patógenos del cultivo. El efecto antagonista de las rizobacterias frente a P. cinnamomi, Colletotrichum sp. y Phomopsis sp. fue probado a través de cultivos duales. El 36% de los aislamientos evaluados mostraron halos de inhibición frente a P. cinnamomi, 36% frente a Colletotrichum sp. y 67% frente a Phomopsis sp. Tres de los aislamientos más promisorios se fermentaron en diferentes medios de cultivo líquidos. Los extractos de los aislamientos ARP5.1 y AED06 a partir de medio mínimo mostraron actividad inhibitoria frente a los patógenos. Los medios suplementados con cloruro de cobre no incrementaron la actividad de los extractos. Estos resultados demostraron la viabilidad de utilizar extractos metabólicos microbianos como una alternativa para controlar in vitro los patógenos de aguacate.
Palabras clave: Persea americana, enfermedades poscosecha, Phytophthora cinnamomi, metabolitos secundarios, rizobacterias.
Introduction
The global production of avocado exceeds 4 million t year-1. In terms of the volume of the fruit and its acreage, avocado holds the fifth largest tropical fruit crop in the world. In 2010, Colombia was ranked fifth in avocado production worldwide (Yabrudy, 2012). Due to its high nutritional value, palatability, versatility, ease of preparation, wide usability in culinary, pharmaceutical and cosmetic industries, this fruit is considered a product with great export potential for Colombia (Alfonso, 2008).
Despite its high production volume, Colombia still relies on imports to meet the domestic demand, which demonstrates the need for increasing avocado production (Alfonso, 2008; Yabrudy, 2012). Moreover, avocado crops are severely affected by diseases such as root rot or dieback, stem-end rot and body rot, caused by P. cinnamomi, Phomopsis sp. and Colletotrichum gloeosporioides, respectively. The latter is responsible for the loss of up to 80% of the harvest in some crops (Demoz and Korsten, 2006; Jianga et al., 2014).
Usually, disease control in avocado productive systems depends on agrochemical applications that have gradually caused important environmental damage and affected human health, turning plant health problems into a chronic threat (Morales, 2009; Romero-Correa et al., 2014). Therefore, the use of compounds from nature, particularly the use of active compounds derived from plant extracts, has been positioning itself as an alternative to overcome these drawbacks (Grahovac et al., 2014). However, the availability of plant material is generally low, making it difficult to reach large scale production and increasing manufacture costs (Subramani and Aalbersberg, 2012; Zabka and Pavela, 2013).
In recent years, the use of microorganisms as a source of bioactive substances has captured the attention of researchers (Bertrand et al., 2014). Microbes have great potential for biological activities and the use of large-scale fermentation processes can reduce production costs of microbe-derived compounds. Antagonists are among plant associated microbes with a big potential to produce bioactive metabolites that can inhibit, partially or totally, the growth of pathogens (Van Eeden and Korsten, 2013). Some of them have been used in biological control systems with very good outcomes (Van Eeden and Korsten, 2013).
Therefore, finding an economically and environmentally viable alternative for obtaining compounds that are useful for the control of plant pathogens is urgent. Thus, the aim of this study was to assess the in vitro capability of antagonistic rhizobacteria associated with avocado crops of controlling and producing bioactive extracellular metabolites against P. cinnamomi, Phomopsis sp. and Colletotrichum sp.
Materials and methods
Culture media and reagents
Trypticase soy agar and broth (TSA and TSB), and Potato-Dextrose Agar (PDA) were purchased from Merck. Sabouraud broth was purchased from BD. Minimal medium (MM) components (200 mL M9 salts: 64.0 g L-1 Na2HPO2·7H2O, 15.0 g L-1 KH2PO4, 2.5 g L-1 NaCl, 5.0 g L-1 NH4Cl; 2.0 mL 1 M MgSO4; 25.0 mL glucose 20%; 0.1 mL 1 M CaCl2; 773 mL water). The solvents were purchased from Merck as well.
Microorganisms
The pathogen strains (Phytophthora cinnamomi, Colletotrichum sp. and Phomopsis sp.) were taken from the plant pathogen bank of Corporación para Investigaciones Biológicas, CIB. Samples for obtaining antagonistic microbes were taken from avocado producing farms located in Anserma (Caldas, Colombia) and in eastern Antioquia (Colombia). Nutritional roots and soil samples were isolated from apparently healthy avocado plants. Five to eight trees were sampled from each farm in a zigzag pattern. The samples were carried in properly labeled plastic bags and stored at 4°C until processed in the laboratory. The roots were washed with sterile distilled water and cut into 0.5 cm pieces. For the endophyte isolation, the roots were washed with distilled water, disinfected with 1% sodium hypochlorite for 1 min and cut into 0.5 cm pieces. The fragments were placed on TSA and incubated at 21°C for 24 to 48 h. After incubation, the single bacterial colonies grown on the surface of the culture medium were sub-cultured in a fresh medium. Each isolate was coded as "AED" in the case of endophytes and as "ARP" in the case of rhizoplane microbes. The purity of the isolates was checked with staining and microscopy techniques.
Antagonism assays
The selection of antagonistic isolates was performed using dual culture assays in petri dishes (Granada et al., 2014). Briefly, petri dishes containing PDA were divided into four quadrants and each one was inoculated at 1 cm from the edge of the plate with a loopful of four different potential antagonists. A plug of the pathogen to be tested, grown during 8 d on PDA, was placed in the middle of the plate. A growth control consisting of the pathogen without any bacteria was performed as well. The inhibition zone around each potential antagonist was measured after 6 d of incubation at 24°C. The degree of inhibition was quantified on a scale from 0 to 3 (0 = no inhibition halo, 1 = halo from 0.1 to1.0 cm, 2 = halo from 1.0 to 2.0 cm, 3 = halo from 2.0 to 4.0 cm). All procedures were conducted in triplicate with a repetition over time.
Fermentations and extracellular metabolite extraction
Pre-inoculum of the bacteria to be fermented were carried out in TSB and incubated for 24 h at 29°C and 200 rpm. Subsequently, 5 mL of the pre-inoculum were added to 125 mL erlenmeyer flasks containing 45 mL of three different media (minimal medium (MM), MM supplemented with copper chloride (1 mM CuCl2), TSB and TSB supplemented with copper chloride (1 mM CuCl2). The incubation conditions were 200 rpm at 29°C for 96 h. After fermentation, the biomass was separated through centrifugation at 8,000 rpm for 5 min, the supernatants were freeze-dried and a solid-liquid extraction was performed using 20 mL of methanol. Methanolic solutions were dried in a vacuum assisted rotary evaporator.
Antimicrobial activity assays of metabolic extracts
Antimicrobial activity assays against Colletotrichum sp., P. cinnamomi and Phomopsis sp. were performed using 96-well microplates (Sanmartín-Negredoet al., 2012; Villamil et al., 2012). A BioRad microplate reader, model 680 XR (BioRad laboratories, Tokyo, Japan), at a 595 nm wavelength, was used. The Colletotrichum sp. inoculum was prepared using agar plugs in sterile distilled water to reach a concentration of 1·104 conidia/mL. The P. cinnamomi and Phomopsis sp. inoculums were prepared by mycelia fragmentation using sterile glass pearls to reach concentrations of 1·104 fragments/mL. Counting was achieved using a hemocytometer. Each plate well had the following added: 50 mL of the pathogen, 100 mL of the Sabouraud broth and 50 mL of the microbial extract re-suspended in sterile distilled water to reach a concentration of 1% w/v. Controls with water instead of the extracts were performed and all of the treatments were carried out in quadruplicate. The microplates were read after 5 d of incubation at 24°C. The data were analyzed using ANOVA with Tukey post-hoc tests with VassarStats online software (available in http://vassarstats.net/anova1u.html).
Results and discussion
Antagonism assays
From the processed samples, 76 bacterial isolates were obtained. All of the pathogens tested showed inhibition halos at different degrees after being antagonized with the 76 bacterial isolates (Fig. 1). The 37% showed inhibition halos against P. cinnamomi, 36% against Colletotrichum sp. and 67% against Phomopsis sp. In the case of P. cinnamomi, 14.5% of isolates were ranked as degree three (3), 10.5% for Colletotrichum sp. and 42% Phomopsis sp. (Tab. 1), which indicates an increased susceptibility of Phomopsis sp. to be inhibited in vitro. It should be noted that several of the isolates showed inhibition zones against more than one pathogen.
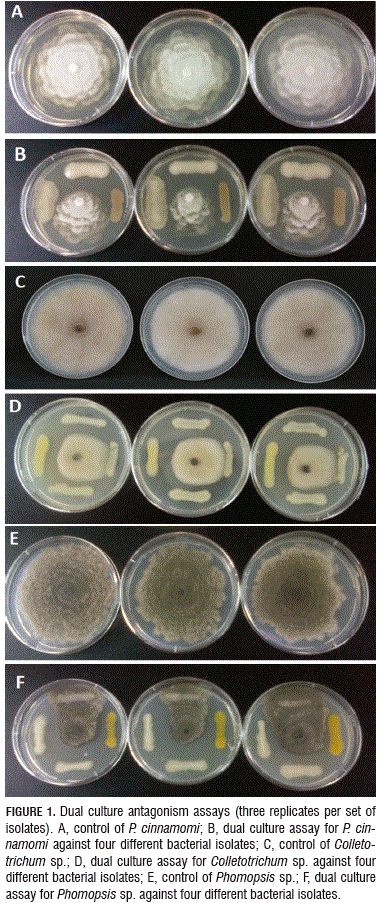
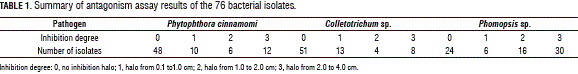
Antimicrobial assays of extracts from fermentations
Isolates AED06, AED26 and ARP5.1 showed the greatest inhibition degree and the broadest spectrum against the tested pathogens. Due to this, they were selected to be grown in broth medium in erlenmeyer flasks.
From the four tested media, the minimal medium (45 mL) was the most appropriate for producing inhibitory metabolites against pathogens used in this study. Although TSB showed a high biomass development, the extracts did not show significant inhibitory activity against pathogens. This may be due to the association of limitation of essential nutrients with the production of secondary metabolites. The depletion of nutrients generates stress responses in microbes, such as motility, secretion of extracellular enzymes, competence for genetic transformation, production of antibiotics or other bioactive compounds (Granada et al., 2014; Thines et al., 2014). Additionally, the media supplemented with CuCl2·2H2O did not show significant activity or diminished biomass production (Carvalho et al., 2010). Nevertheless, several authors have reported a boost in antibiotic production and a positive regulation of secondary metabolism of various types of microorganisms when heavy metals such as cooper are added to culture media (Paul and Banerjee, 1983; Tunac and Mcdaniel, 1985; Jafarzade et al., 2013).
Conversely, copper is known to participate in the degradation of nucleic acids through destabilization and oxidative or hydrolytic breakdown of the double helix, possibly explaining the inhibition observed in the supplemented fermentations (Cervantes and Gutiérrez-Corona, 1994; Weinberg, 1997). However, copper's toxicity depends on the environmental conditions, concentration and type of microbes in the environment (Lippert, 1992).
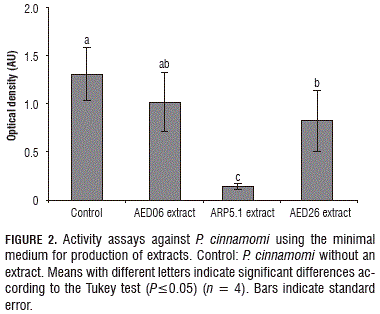
Extract from the ARP5.1 isolate showed the highest inhibition activity against all of the tested pathogens, which was evidenced by the low optical density. Lower optical densities involve a small development of biomass; i.e. inhibition or less growth of the evaluated microbes. The characteristics of the limiting conditions of salts and glucose in the minimal medium probably stimulated the production of molecules involved in the antagonistic mechanisms (Ruyters et al., 2013). The growth of the P. cinnamomi treated with the AED26 extract was also significantly lower (P≤0.05), as compared with the control (Fig. 2), but it did not have an effect on the other tested pathogens. This is a surprising result because this isolate was selected due to its inhibition halos in dual culture tests. This is probably owing to the culture media used that impairs the production of bioactive metabolites on this isolate. Moreover, it has been reported that some bacteria are capable of producing such compounds in response to the presence of other microorganisms or in response to aspects of the environment (Ryan and Dow, 2008). Therefore, in the absence of competitors, compounds with a biocidal capacity are not produced by the antagonist and further studies must be performed on this type of isolates.
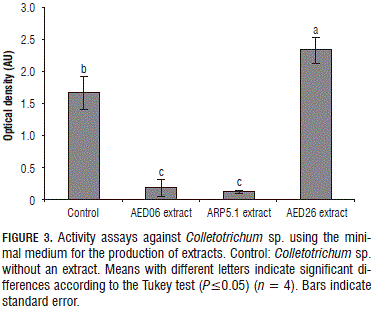
The AED06 extract showed high inhibition against Colletotrichum sp. and Phomopsis sp., but a low effect on P. cinnamomi (Figs. 3 and 4). P. cinnamomi is not considered a fungus, but is a pathogen of the Oomycete class; this might imply that metabolites produced by AED06 have a narrow spectrum and are only active against true fungi. Conversely, metabolites produced by ARP5.1 have a broader spectrum and their mode of action might be able to kill the different tested pathogens generally.
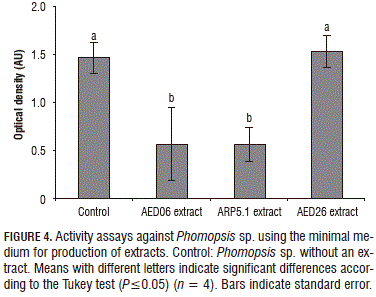
This study showed that healthy avocado trees are associated with microbes capable of in vitro inhibition against the growth of pathogens such as P. cinnamomi, Colletotrichum sp. and Phomopsis sp., and that one of the mechanisms for inhibition is the production of secondary metabolites. Furthermore, the minimal medium represented the most suitable substrate for the production of inhibitory extracts by the tested bacteria. Additionally, it was observed that the potential of a microbe to produce inhibitory metabolites not only depends on what is evidenced from dual culture assays using petri dishes, but may also possibly be a response to the presence of other competing microbes. Thus, the production of said metabolites in an axenic liquid medium may be limited when there are not any competing microbes.
Acknowledgement
The authors would like to thank the Institución Universitaria Colegio Mayor de Antioquia, especially the Faculty of Health Sciences, for funding this research.
Literature cited
Alfonso B, J.A. 2008. Manual técnico del cultivo de aguacate Hass (Persea americana L.). Fundación Hondureña de Investigación Agrícola (FHIA), La Lima, Honduras. [ Links ]
Bertrand, S., N. Bohni, S. Schnee, O. Schumpp, K. Gindro, and J.L. Wolfender. 2014. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 32, 1180-1204. Doi: 10.1016/j.biotechadv.2014.03.001 [ Links ]
Carvalho, A.L.U., F.H.P.C. Oliveira, R.L.R. Mariano, E.R. Gouveia, and A.M. Souto-Maior. 2010. Growth, sporulation and production of bioactive compounds by Bacillus subtilis R14. Braz. Arch. Biol. Technol. 53, 643-652. Doi: 10.1590/S1516-89132010000300020 [ Links ]
Cervantes, C. and F. Gutiérrez-Corona. 1994. Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol. Rev. 14, 121-137. Doi: 10.1111/j.1574-6976.1994.tb00083.x [ Links ]
Demoz, B.T. and L. Korsten. 2006. Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stem-end rot pathogens. Biol. Control 37, 68-74. Doi: 10.1016/j.biocontrol.2005.11.010 [ Links ]
Grahovac, J., M. Grahovac, J. Dodic, B. Bajic, and J. Balaz. 2014. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicus. Crop Prot. 65, 143-152. Doi: 10.1016/j.cropro.2014.07.020 [ Links ]
Granada G, S.D., A. Rueda L., and C.A. Peláez. 2014. Antimicrobial activity of extracellular metabolites from antagonistic bacteria isolated from potato (Solanum phureja) crops. Summa Phytopthol. 40, 212-220. Doi: 10.1590/0100-5405/1953 [ Links ]
Jafarzade, M., N.A. Yahya, F. Shayesteh, G. Usup, and A. Ahmad. 2013. Influence of culture conditions and medium composition on the production of antibacterial compounds by marine Serratia sp. WPRA3. J. Microbiol. 51, 373-379. Doi: 10.1007/s12275-013-2440-2 [ Links ]
Jianga, J., H. Zhaia, H. Li, Z. Wang, Y. Chen, N. Hong, G. Wang, G.N. Chofong, and W. Xu. 2014. Identification and characterization of Colletotrichum fructicola causing black spots on young fruits related to bitter rot of pear (Pyrus bretschneideri Rehd.) in China. Crop Prot. 58, 41-48. Doi: 10.1016/j.cropro.2014.01.003 [ Links ]
Lippert, B. 1992. From cisplatin to artificial nucleases - the role of metal ion-nucleic acid interactions in biology. Biometals 5, 195-208. Doi: 10.1007/BF01061218 [ Links ]
Morales, J. 2009. Enfermedades de importancia económica en el cultivo de aguacate. pp. 15-31. In: Libro de Resúmenes, III Congreso Latinoamericano del Aguacate. Medellin, Colombia. [ Links ]
Paul, A.K. and A.K. Banerjee. 1983. Determination of optimum conditions for antibiotic production by Streptomyces galbus. Folia Microbiol. 28, 397-405. Doi: 10.1007/BF02879489 [ Links ]
Romero-Correa, M.T., R. Villa-Gómez, E. Castro-Mercado, and E. García-Pineda. 2014. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 87, 32-41. Doi: 10.1016/j.pmpp.2014.05.003 [ Links ]
Ruyters, S., P. Salaets, K. Oorts, and E. Smolders. 2013. Copper toxicity in soils under established vineyards in Europe: a survey. Sci. Total Environ. 443, 470-477. Doi: 10.1016/j.scitotenv.2012.11.001 [ Links ]
Ryan, R.P. and J.M. Dow. 2008. Diffusible signals and interspecies communication in bacteria. Microbiology 154, 1845-1858. Doi: 10.1099/mic.0.2008/017871-0 [ Links ]
Sanmartín-Negredo, P., X. López, M.P. Pemberthy, D. Granada-Sair, and E.A. Rueda-Lorza. 2012. Análisis del modo de acción de la capacidad antagónica de Trichoderma asperellum sobre Colletotrichum gloeosporioides y Fusarium sp. Rev. Tumbaga 2, 29-49. [ Links ]
Subramani, R. and W. Aalbersberg. 2012. Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol. Res. 167, 571-580. Doi: 10.1016/j.micres.2012.06.005 [ Links ]
Thines, E., H. Anke, and R.W.S. Weber. 2004. Fungal secondary metabolites as inhibitors of infection-related morphogenesis in phytopathogenic fungi. Mycol. Res. 108, 14-25. Doi: 10.1017/S0953756203008943 [ Links ]
Tunac, J.B. and L.E. McDaniel. 1985. Effect of phosphate and copper on the fermentation of hydroheptin. Appl. Environ. Microb. 50, 1192-1195. [ Links ]
Van Eeden, M. and L. Korsten. 2013. Factors determining use of biological disease control measures by the avocado industry in South Africa. Crop Prot. 51, 7-13. Doi: 10.1016/j.cropro.2013.03.011 [ Links ]
Villamil C., J.E., J.O. Blanco V., and S.E. Viteri R. 2012. Evaluación in vitro de microorganismos nativos por su antagonismo contra Moniliophthora roreri Cif. & Par. en cacao (Theobroma cacao L.). Rev. Fac. Nal. Agr. Medellin 65, 6305-6315. [ Links ]
Weinberg, E.D. 1997. Mineral element control of microbial secondary metabolism. pp. 289-316. In: Weinberg, E.D. (ed.). Microorganisms and minerals. Marcel Dekker, New York, NY. [ Links ]
Yabrudy V., J. 2012. El aguacate en Colombia: Estudio de caso de los Montes de María, en el Caribe colombiano. Documentos de Trabajo de Economía Regional 171, 1-45. [ Links ]
Zabka, M. and R. Pavela. 2013. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 93, 1051-1056. Doi: 10.1016/j.chemosphere.2013.05.076 [ Links ]














