Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.33 no.2 Bogotá May/Aug. 2015
https://doi.org/10.15446/agron.colomb.v33n2.49858
Doi: 10.15446/agron.colomb.v33n2.49858
1 Agencia para el Desarrollo Economico Local de la cienaga de Zapatosa (ADEL Zapatosa). Valledupar (Colombia)
2 Agronutrisalud group, Faculty of Health, Universidad de Santander (UDES). Valledupar (Colombia)
3 Observatorio del Caribe Colombiano. Cartagena (Colombia)
4 Motilonia Research Center, Corporacion Colombiana de Investigacion Agropecuaria (Corpoica). Codazzi (Colombia). atofino@corpoica.org.co
Received for publication: 27 March, 2015. Accepted for publication: 30 June, 2015.
Abstract
The sustainable expansion of bean cultivations requires technologies that do not limit their phyto-recovering properties. Therefore, the objective of this study was to propose agronomic management of conservation for bean cultivation considering the microbiological characteristics of two mega-environments of the Colombian Caribbean coast and the compatibility between agrochemicals and bioinputs. The methodology included rhizospheric microbe population counts, identification of phytopathogenic fungi in plant tissues and soils, compatibility studies of pesticides with biocontrollers, and determination of residual contents of pesticides in bean seeds. The microbial populations corresponded to those previously registered for the lower tropics, but with quantitative differences in the genera. Phytophthora, Colletotrichum and Fusarium were registered in the humid Caribbean, while Colletotrichum and Curvularia affected crops in the dry Caribbean. The Beauveria bioinput was not compatible with the evaluated agrochemicals, while Trichoderma was compatible with chlorpyrifos, thiabendazole and oxycarboxin. Metarhizium was compatible with glyphosate and oxycarboxin at 10% of the recommended dose. Lindane residues were found in the beans harvested at three of the studied locations. The combined use of agrochemicals and bioinputs on bean crops is feasible as long as the time of application of the latter is made according to the half-life of the chemical and the organic matter content of the soil is increased.
Key words: sustainable agriculture, entomopathogens, biocontrollers, Phaseolus vulgaris L.
Resumen
La expansión sostenible del cultivo de fríjol requiere el desarrollo de tecnologías que no limiten sus propiedades fito-recuperadoras. En consecuencia, el objetivo del estudio fue proponer un manejo agronómico de conservación del cultivo de fríjol, considerando las características edafológicas y microbiológicas de dos mega-ambientes del Caribe colombiano y, la compatibilidad entre agroquímicos y bioinsumos. La metodología incluyó recuento de poblaciones microbianas rizosféricas; identificación de hongos fitopatógenos en tejido vegetal y suelo; estudio de compatibilidad de pesticidas con biocontroladores; y determinación del contenido de residuos de pesticidas en semillas de fríjol. Las poblaciones microbianas correspondieron a los registrados previamente para suelos del trópico bajo, pero con diferencias cuantitativas de géneros. Phytophthora, Colletotrichum y Fusarium fueron registrados en el Caribe húmedo mientras se identificaron Colletotrichum y Curvularia en el Caribe seco. El bioinsumo Beauveria no fue compatible con los agroquímicos ensayados, mientras que Trichoderma fue compatible con clorpirifos, tiabendazol y oxicarboxin, y Metarhizium con glifosato y oxicarboxin al 10% de la dosis recomendada. Se identificó la presencia de residuos de lindano en semillas cosechadas en tres localidades. El uso combinado de agroquímicos y bioinsumos en el cultivo de fríjol es factible, siempre y cuando el momento de aplicación del último sea acorde a la vida media del químico y se incremente el contenido de materia orgánica del suelo.
Palabras clave: agricultura sustentable, entomopatógenos, biocontroladores, Phaseolus vulgaris L.
Introduction
The bean is one of the crops with the greatest direct consumption in Colombia and worldwide and has a national annual production of 71,467 t, cultivated in 34,032 ha (DANE, 2011). It plays an important role from an environmental standpoint, given its nitrogen fixating properties, which is why it has been used extensively in sustainable agriculture, including the recuperation of degraded soils due to intensive agriculture, contamination by chemicals, and mining, among other anthropogenic interventions (García, 2013).
However, the possible phyto-recuperative actions of the bean are limited by traditional agronomic management, characterized by the indiscriminate application of chemicals, socioeconomically justified by the necessity of increasing production and food security (Xie et al., 2011). Such management deteriorates the physicochemical and biological properties of the soil, jeopardizing its quality and thereby being the third most common cause of desertification (Pava, 2011). This is problematic given the high use of agrochemicals in comparison to organic products on the Colombian Caribbean coast (DANE, 2011).
Additionally, the agrochemicals most frequently used in the region are nonselective and, therefore, have a low effect on pathogen and pest control. The lack of technical assistance for farmers limits the identification of causal agents, resulting in repeated applications of agrochemicals in order to control phytosanitary problems (Jojoa and Salazar, 2011; Tofiñoet al., 2012). Because of this, an unfavorable environmental impact is generated due to a reduction in the beneficial rhizospheric microbiota that is qualitatively and quantitatively sensitive to the action of diverse pesticides. This microbiota intervenes in the productive process of beans, such as growth promoters, entomopathogens, and antagonists, and alters the metabolites secreted by plants that constitute an important source of nutrients for diverse functional groups of microorganisms (Soto et al., 2010).
In beans, up to 20 applications of insecticides and six to eight applications of fungicides have been reported, resulting in an increased risk of resistance to the active substances (Morros and Pire, 2003; Rodríguez et al., 2003). Additionally, high application frequencies lead to elevated contents of pesticide residues in products that are highly carcinogenic, mutagenic and may affect the central nervous system (Roubos et al., 2014). This justifies the development of alternative integral management systems for the sustainable production of food products (Gallego et al., 2010; Santos et al., 2013).
Especially on the Colombian Caribbean coast, the Integrated Pest and Disease Management (IPDM) is limited because, among other reasons, of the lack of compatibility studies between bioinputs and the agrochemicals commonly used in vegetables and the lack of information regarding the agroecological distribution of phytopathogenic fungus in the soil that may attack the bean (Santos et al., 2013; Tofiño, 2013).
This study was developed with the objective of finding basic elements that contribute to the formulation of a sustainable productive model for bean cultivation, based on the edapho-microbiological characteristics of the Colombian Caribbean coast and the compatibility between agrochemical and biological inputs, that will allow for an improvement in bean production as well as the quality of soils.
Materials and methods
Location. Samples were collected from two commercial production zones, selected for their vocation for bean production and problems with desertification in the humid Caribbean (Cerete [department of Cordoba] and Carmen de Bolivar [department of Bolivar]), and from seven locations in the dry Caribbean (Codazzi, Manaure, La Jagua, Tamalameque and Valledupar [department of Cesar]; San Juan [department of La Guajira]; and Sevilla, [department of Magdalena]). The collection was done during the rainy season of 2013 for all of the samples except the samples from Codazzi, which were taken during the rainy season of 2014.
Soil sampling. Soil samples of 100 g were collected from each of five points, at 0 to 15 cm in depth, for a total of 500 g ha-1 of sample in the cultivated locations. The samples were stored in airtight bags, refrigerated at 4°C, and transported to the Microbiology Laboratory at Universidad de Santander.
Plant tissue sampling. The plant tissue collection was done with convenience sampling by identifying the roots, stems and leaves of the bean plants with fungal symptomatology that corresponded to the genotypes developed by CIAT that were used in agronomic studies by Corpoica at the time. To eliminate microorganisms from the surface, the sampled material was cut into pieces and washed in distilled water for 20 min, disinfected with alcohol (30%) and hypochlorite (0.05%) solutions for 2 and 1 min, respectively, and dried in a laminar air-flow bench following the protocol of Tofiñoet al. (2012).
Quantification of rhizospheric soil microorganisms and identification of fungal genera. Bacterial, fungal and actinomycete counts were carried out on each of the collected soil samples, measured as colony forming units per gram (cfu/g) of soil using 10 fold serial dilution with distilled water and subsequent inoculation by pour plate in nutrient agar for the bacteria and potato dextrose agar for the fungi. The fungal genera were identified by macro-microscopic observation and taxonomic keys and the relative frequency of each genus was compared to the number of samples collected per recorded mega-environment (Tofiñoet al., 2012).
Characterization of phytopathogenic fungi. The previously prepared plant tissue was incubated in oxytetracyclin glucose yeast agar at 28°C for 5 d. After the incubation, the macroscopic growth and microstructures of the isolated colonies were studied using lactophenol cotton blue staining, optic microscopy and taxonomic guides in order to determine their genera (Tofiñoet al., 2012).Thereafter, the incidence of phytopathogenic fungi in relation to the mega-environment cultivated with bean was calculated.
Agrochemical-bioinput compatibility test. The methodology proposed by Reyes et al. (2012) was modified (agrochemical doses higher than the recommended dose were not included) and applied. Pure cultures of Trichoderma (strain PB2011) from the strain collection of Universidad de Santander and formulations of bioinsecticides based on Metarhizium anisopliae (Metaril®) and Beauveria bassiana (Baubasil®) from the brand Fungicol® were used. This study was performed using the agrochemicals frequently used in bean cultivation in Cesar, according to previous surveys applied by technical assistants (Tofiñoet al., 2012). Three herbicides were applied: paraquat (Gramoxone®), glufosinate ammonium (Finale®), and glyphosate (Roundup® Activo SL). Additionally, one insecticide was used: chlorpyrifos (Lorsban⢠líquido 4EC), as well as four fungicides: mancozeb (Invezeb® 80WP), carbendazim (Derosal® 500SC), oxycarboxin (PlantVax®75%), and thiabendazole (Mertect® 500SC). Three concentrations were applied: high (recommended dose), medium (50% of the recommended dose), and low (25% of the recommended dose for paraquat and glufosinate ammonium and 10% of the recommended dose for the remaining agrochemicals) (Tab. 1).
The mycelial growth and spore formation evaluation was done through inoculation of the active fungal growth zone in Petri dishes (50 mm) with oxytetracyclin glucose yeast agar (OGY), supplemented with the desired agrochemical. The dishes were incubated in darkness at 28±5°C and observed every 72 h for mycelial growth and every 7 d for spore formation. Thereafter, the diameter of the colonies was measured to determine the effect of the agrochemicals on the growth compared to the control (OGY without agrochemicals). The three-level toxicity scale cited by Reyes et al. (2012) was used to classify the agrochemicals: level 1 - compatible, less than 10% effect on the mycelial growth (EMG); level 2 - moderately compatible, 10 to 30% EMG; level 3 - not compatible, more than 30% EMG.
Weed control evaluation. An application of glufosinate ammonium in combination with an organic herbicide based on fermented aromatic plant extracts (ecoredudoq) was done in Codazzi during the pre-seeding. The treatment combinations were as follows: control (manual weed control), 25F-75E (25% glufosinate ammonium + 75% ecoredudoq), 50F-50E (50% glufosinate ammonium + 50% ecoredudoq), 75F-25E (75% glufosinate ammonium + 25% ecoredudoq), 100F (100% glufosinate ammonium) and 100E (100% ecoredudoq) based on the recommended concentrations. After the treatments, the effectiveness of the weed control was evaluated and the rizospheric microorganism counts and fungal genera identification were performed following the previously described methodology.
Pesticide residue analysis in the bean seeds. Seeds from Codazzi, La Paz, Pueblo Bello and Manaure (Cesar, Colombia), as well as a control from Codazzi without an agrochemical application, were analyzed to evaluate the impact of agrochemical management in 2013. The samples were collected and prepared for analysis at the pesticide analysis laboratory, Fytolab (Cota, Colombia). Gas chromatography - tandem mass spectrometry (GC-MSMS) and liquid chromatography-tandem mass spectrometry (LC-MSMS) techniques were used, both methods are ISO 17025:2005 certified for fruits, vegetables, and cereals.
Statistical analyses. Descriptive statistics, such as the calculation of means and standard deviations for quantitative variables and frequencies for qualitative variables, were used for the characterization of the soil microbes. In the bioinput-agrochemical compatibility study, a completely randomized design with two factors (product and dose) and three repetitions were used. A randomized block design with three repetitions was used to evaluate the efficiency of the herbicides in the field. An analysis of variance followed by a Tukey test at the 5% level of probability was applied using SPSS v.17 for Windows.
Results and discussion
Microbiological analysis
In general, all of the locations cultivated with beans generated population densities, measured as ufc/g of soil, that were similar to those previously reported for tropical soils (Garau et al., 2012). However, the diversity of the identified genera was low (between 2 and 6 genera with an average of 4.2), as compared to results obtained in other studies where more than 6 genera were identified (Tab. 2) (Arenas et al., 2005).
The quantitative equilibrium of the rhizospheric microbe groups identified in the evaluated environments showed a direct positive relationship with the pH levels, which on average were 6.02±0.5, being higher for the bacteria (r=0.7) and lower for the fungi (r=0.3). These results coincide with those found by Garau et al. (2012).
The low diversity of the fungal genera was associated with the inadequate levels of organic matter identified in some of the locations included in the studies. Concentrations of 0.81 to 2.9% were measured at locations where a maximum of four genera were identified, which, in combination with the indiscriminate application of herbicides, favors the development of pathogens in the soil (Tofiñoet al., 2012; Ahemad, 2014).
In addition, no significant differences were found in the prevalence of genera between the mega-environments (P=0.896) (Tab. 3). The genera Penicillium, Aspergillus sp., and Rhizopus have been identified previously as rhizospheric microbiota in bean cultivations, fulfilling functions as saprophytes and phosphate solubilizers and, in the case of Aspergillus, causing seed rotting (Peña-Betancourt and Conde-Martínez, 2012). Various species of Phytophthora and Macrophomina cause disease in the pod, root, and stem (Ulacio et al., 2012). Trichoderma and Paecilomyces act as antagonists to pathogens and control pests (Mendoza et al., 2013; Martínezet al., 2013). Fusarium and Cladosporium have received attention due to their isolation in plant tissues with symptoms of radical wilting and anthracnose (El-Mohamedy and Abd Alla, 2013); the former can cause production losses of between 17 and 45% (Garcés-Fiallos, 2013). In plant tissue samples of Sevilla beans of the varieties SCO487 and SCO611, Curvularia and Colletotrichum were found (Tab. 4), infecting the transition zone between the root and stem. Colletotrichum is found worldwide and has been reported to cause anthracnose, a disease that is considered to be the most limiting in bean cultivations and that can cause yield losses of between 38 and 95% (Gallego et al., 2010).

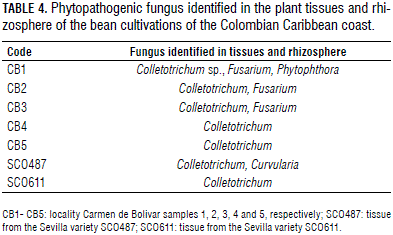
The colonization of three phytopathogens, infecting individually and/or in cooperation, was observed in the bean tissue cultivated in Carmen de Bolivar (Tab. 4). For example, in sample CB1, Colletotrichum sp., Fusarium, and Phytophthora were observed. The cooperative infection of these phytopathogens causes damping-off and should be managed through selective fungicides (El-Mohamedy and Abd Alla, 2013).
The greater prevalence of pathogens identified in the bean plant tissue, the lower proportion of beneficial fungi in the rhizosphere in the humid environment, and a low average number of fungal genera in both mega-environments indicated the need to add bioinputs and increase beneficial microorganisms, such as Trichoderma and Paecilomyces. These biocontrollers have an antagonistic effect on the phytopathogens Colletotrichum sp., Fusarium and Phytophthora, identified in the dry as well as the humid Caribbean (Rojanet al., 2010; Taj and Kumar, 2012) and should be used as a component of IPDM, combined with selective agrochemicals with a low toxicity level, to avoid an inhibitory effect on the biocontrollers and other microbial genera found in beans (Herrera et al., 2012). In general, the quantitative and qualitative characteristics of the biodiversity of microorganisms will improve and contribute to the development of a suppressive rhizosphere (Lang et al., 2012; Yadav et al., 2013), conserved through sustainable management with the addition of organic matter to reduce the environmental impact caused by the pesticides (Quintero, 2011; Tofiñoet al., 2012).
Bioinput-agrochemical compatibility
The evaluated doses of paraquat and glufosinate ammonium affected the development of the biocontrollers, with significant differences in the radial growth (P=0.00). However, according to the EMG scale, the glufosinate ammonium, at the concentration of 250 mL/200 L, was moderately compatible with Trichoderma and Beauveria with an EMG lower than 30%. This compatibility level is associated with a lower toxicity level of the product according to categories of the Environmental Protection Agency. Although this dose is not effective in the field, the specifications regarding the product's half-life (MAVDT, 2011) indicate the possibility of applying the higher dose and, after 6 to 11 d, applying the bioinput. The inhibitory effect of paraquat on B. bassiana at more than 50% has been shown previously (Pandey et al., 2008) (Tab. 5).
No significant differences were found in the growth of the three microorganisms between the low dose of glyphosate and the control (P=0.85, and P=0.32). Moreover, the low dose corresponded to level 1 compatibility. Therefore, applications of glyphosate and bioinputs should be done with a 20 to 60 d difference (UNL, 2010).
It was found that thiabendazole and chlorpyrifos allowed for the growth of Trichoderma in all of the concentrations with a compatibility corresponding to level 1 or 2 (Tab. 5). However, similar to the findings of Muhammad et al. (2010), the growth of Beauveria and Metarhizium was inhibited by these two agrochemicals.
The fungicides mancozeb and carbendazim inhibited the growth of the fungi with significant differences between them and the control (P=0.00), with a level 3 compatibility. Oxycarboxin presented a high level of compatibility with Metarhizium according to the toxicity scale and did not show a significantly different growth between the low concentration and the control (P=0.646). However, the growth of B. bassiana was inhibited by the evaluated fungicides but with growth at the low dose of oxycarboxin.
Herrera et al. (2012) evaluated the growth of various strains of Trichoderma using recommended concentrations of mancozeb for the control of Phytophthora, finding that both the native and mutant strains were highly sensitive to the fungicide. Likewise, its inhibitory effect on B. bassiana growth has been reported previously (Gangwar, 2013).
Management plan based on microbiological analyses and compatibility tests
According to the obtained results (Tab. 6) and previous reports regarding the inhibition of herbicides on the saprophytes Mucor, Aspergillus and Penicillium (Zain et al., 2013), which are frequently found in the study areas, the order of inhibition of the beneficial rhizospheric microbiota and bioinputs by herbicides can be established as asparaquate>glufosinate ammonium=glyphosate (Santoro, 2014). Trichoderma was compatible with glyphosate at concentrations 10 times lower than the recommended dose and moderately compatible with glufosinate ammonium at the same concentration. However, the efficacy of the agrochemicals toward weeds was greatly reduced when a low concentration was applied; therefore, it is advisable to use 100% of the recommended dose, considering its half-life, when programming applications of bioinputs. Biological products should therefore be applied 20 and 11 d after the application of glyphosate and glufosinate ammonium, respectively, in order to avoid effects on the growth of entopathogenic and antagonistic fungi. Metarhizium was only compatible with glyphosate and oxycarboxin at 10% of the recommended dose, which suggests that this bioinput can be applied 20 and 44 d after the application of the respective agrochemicals.
Given the above, the field application can be 1 L of glufosinate ammonium/200 L water per ha or 2 L of glyphosate/200 L water per ha and the addition of the bioinputs Trichoderma and Metarhizium can occur after 6 to 11 d in the case of glufosinate ammonium and after 20 d in the case of glyphosate.
Likewise, given their high compatibility, it is recommended to apply chlorpyrifos and oxycarboxin together with bioinputs based on Trichoderma (ANLA, 2011; MAVDT, 2007).
Beauveria bassiana only showed compatibility with low doses of glyphosate, which limits its inclusion in the IPDM of beans. Therefore, it is recommended to use Metarhizium given its high effectiveness and compatibility with the mentioned agrochemicals.
Field trials
No statistical difference (P=0.069) was found between the manual weed eradication (99.3% efficacy) and the application of glufosinate ammonium at 100% of the recommended dose (97.3% efficacy). However, significant differences were found between these and the combinations of the two herbicides, where the individual application of ecoredudoq had the lowest efficacy (9.3%). In order to reach weed control, the combination of at least 50% of the recommended dose of glufosinate ammonium is needed as ecoredudoq by itself does not present an herbicidal effect on succulent weeds such as Portulaca oleracea, which was predominant in the evaluated field (Tofiño, 2000). Additionally, there was a reduction in the bacterial population in the treatments that included ecoredudoq, which may favor the growth of Phytophthora.
Pesticide residue detection in the bean seeds
Concentrations of lindane surpassing the maximum residue level (MRL) were found in samples obtained in Codazzi, Manaure, and La Paz in the 2013 crop (MRL = 0.0100 mg Kg-1 according to the Ministerio de la Protección Social, 2007) (Tab. 7). This product was not included in the management plan of the cultivation during this study, which indicates its high residual effect. The use of lindane is prohibited in 52 countries, including Colombia, because it is bioaccumulative and a carcinogenic persistent toxin, detected worldwide in the air, water, snow, tissues and soil with a half-life in the latter of one to two years (Quintero, 2011; Maliszewska-Kordybach et al., 2014). The accumulation of pesticide residues in the analyzed bean seeds is associated with a plant´s capacity to extract the chemical from the soil and the low levels of organic matter in the area (Robles-González et al., 2006).
Chlorpyrifos, thiabenzadole, and carboxine were identified at levels lower than the maximum permitted and, due to their low residual levels and high compatibility with the studied bioinputs, were included in the management plan.
Conclusions
In general, all of the evaluated samples showed favorable microbial populations for bean growth, corresponding to the principal population groups of tropical soils. However, on a qualitative level, the average diversity of the fungal genera was low and in direct proportion to the decrease of the physicochemical quality of the soil in terms of organic matter and pH, regardless of the mega-environment. As a consequence, these unfavorable soil characteristics promote susceptibility to disease because of the lack of a suppressive rhizosphere. This was shown by the higher prevalence of pathogenic microorganisms over beneficial ones, indicating the need to use pesticides, such as chlorpyrifos, thiabendazole, oxycarboxin, and glyphosate, that have a low effect on the development of the microbiota present in the soils used for bean cultivation in the Caribbean region. Such pesticides permit the use of biocontrollers such as Trichoderma and Metarhizium, applied according to the half-life of the chemical, in order to avoid major deterioration of vulnerable Caribbean soils. In addition, organic fertilizers are recommended in order to increase the organic matter content of soils, which favors the suppressive rhizospheric population and the development of the crop.
The deficient health of soils dedicated to bean cultivation on the Caribbean coast justifies the previous recommendations, including techniques used in organic agriculture and IPDM with compatible chemical and biological products, in order to benefit the productivity and the phyto-recovering properties of the plants.
Beauveria bassiana was not compatible with the evaluated agrochemicals and should not be used in integrated management. This indicates the need of further studies on native strains that are better adapted to the climatic and edaphic conditions and the agronomic management of the studied area.
The accumulation of highly toxic pesticides in bean seeds, favored by the phytoextractive properties of the plants and the physicochemical properties of the soil, is an indicator of the need of applying sustainable management that includes agrochemicals with a low residual effect.
Literature cited
Ahemad, M. 2014. Growth suppression of legumes in pyriproxyfen stressed soils: a comparative study. Emir. J. Food Agric. 2, 66-72. [ Links ]
ANLA, Autoridad Nacional de Licencias Ambientales. 2011. Resolución No. 73, por la cual se emite un dictamen técnico ambiental para el producto formulado Lorsban* 4 EC, a partir del ingrediente activo grado técnico clorpirifos. República de Colombia, Bogota. [ Links ]
Arenas, J., F.G. Carpio, and J.J. Guillermo. 2005. Flora fúngica de la rizósfera de Phaseolus lunatus «pallar» en Ica, Perú. Rev. Peru Biol. 12, 441-444. [ Links ]
DANE, Departamento Administrativo Nacional de Estadística. 2011. Resultados Encuesta Nacional Agropecuaria ENA. Ministerio de Agricultura y Desarrollo Rural, Bogota. [ Links ]
El-Mohamedy, R.S.R. and M.A. Abd Alla. 2013. Bio-priming seed treatment for biological control of soil borne fungi causing root rot of green bean (Phaseolus vulgaris L.). J. Agric. Technol. 9, 589-599. [ Links ]
Gallego G., C., G.A. Ligarreto M., L.N. Garzón G., O.A. Oliveros G., and J.L. Rincón R. 2010. Rendimiento y reacción a Colletotrichum lindemuathianum en cultivares de fríjol voluble (Phaseolus vulgaris L.). Rev. Fac. Nal. Agr. Medellin 63, 5477-5488. [ Links ]
Gangwar, G.P. 2013. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with chemicals and bio-pesticides. Ann. Plant Protection Sci. 21, 360-363. [ Links ]
Garau, G., P. Castaldi, S. Deiana, P. Campus, A. Mazza, P. Deiana, and A. Pais. 2012. Assessment of the use potential of edible sea urchins (Paracentrotus lividus) processing waste within the agricultural system: influence on soil chemical and biological properties and bean (Phaseolus vulgaris) and wheat (Triticum vulgare) growth in an amended acidic soil. J. Environ. Manag. 109, 12-18. Doi: 10.1016/j.jenvman.2012.05.001 [ Links ]
Garcés-Fiallos, F.R. 2013. Cuantificación de enfermedades en líneas promisorias y variedades de fréjol en Quevedo, Ecuador. Biotecnol. Sector Agropec. Agroind. 11, 196-207. [ Links ]
García G., A. 2013. Estudio de las alteraciones inducidas por imazamox en judía y veza para la selección de simbiosis Rhizobium-leguminosa tolerantes. PhD thesis. Department of Sciences, Editorial Universidad de Granada, Granada, Spain. [ Links ]
Herrera, R., D. Núñez, N. Romero, X. Besoain, L.M. Pérez, and J. Montealegre. 2012. Sensitivity of wild-type and mutant Trichoderma harzianum strains to fungicides. Cienc. Inv. Agr. 39, 569-576. Doi: 10.4067/S0718-16202012000300016 [ Links ]
Jojoa B., C.J. and C. Salazar G. 2011. Evaluación in vitro de insecticidas biorracionales para el control de Agrotisipsilon Hüfnagel. Rev. Cienc. Agr. 28, 53-63. [ Links ]
Lang, J., J. Hu, W. Ran, Y. Xu, and Q. Shen. 2012. Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol. Fert. Soils 48, 191-203. Doi: 10.1007/s00374-011-0617-6 [ Links ]
Maliszewska-Kordybach, B., B. Smreczak, and A. Klimkowicz-Pawlas. 2014. Evaluation of the status of contamination of arable soils in Poland with DDT and HCH residues: national and regional scales. Pol. J. Environ. Stud. 23, 139-148. [ Links ]
Martínez, B., D. Infante, and Y. Reyes. 2013. Trichoderma spp. y su función en el control de plagas en los cultivos. Rev. Protección Veg. 28, 1-11. [ Links ]
MAVDT, Ministerio de Ambiente, Vivienda y Desarrollo Territorial. 2007. Resolución No. 464, por la cual se otorga una licencia ambiental para la importación del ingrediente activo grado técnico Oxicarboxin y sus formulaciones Plantvax® 5% gr y Plantvax 75%, y se toman otras determinaciones. República de Colombia, Bogota. [ Links ]
MAVDT, Ministerio de Ambiente, Vivienda y Desarrollo Territorial. 2011. Resolución No.27, por la cual se emite un dictamen técnico ambiental para el producto formulado Destierro® SL, a partir del ingrediente activo grado técnico glufosinato de amonio. República de Colombia, Bogota. [ Links ]
Mendoza, G.A.T., J.H. Wilson, and J.C. Colina. 2013. Efecto de Trichoderma atroviride, Trichoderma harzianum y Trichoderma viride sobre huevos de Meloidogyne sp. en condiciones de laboratorio. Rebiolest 1, e65. [ Links ]
Ministerio de la Protección Social. 2007. Resolución No. 2906, por la cual se establecen los Límites Máximos de Residuos de Plaguicidas - LMR en alimentos para consumo humano y en piensos o forrajes. República de Colombia, Bogota. [ Links ]
Morros, M.E. and A. Pire. 2003. Evaluación participativa de materiales promisorios de vainita Phaseolus vulgaris L. en las zonas altas del estado Lara. Rev. Fac. Agron. (LUZ) 20, 21-33. [ Links ]
Muhammad, R.A., H.B. Muhammad, A. Muhammad, A. Muhammad, and T.S. Shahbaz. 2010. Compatibility of entomopathogenic fungi, Metarhizium anisopliae and Paecilomyces fumosoroseus with selective insecticides. Pak. J. Bot. 42, 4207-4214. [ Links ]
Pandey, AK., K.R. Kanaujia, and I. Varshneya. 2008. Effect of different herbicides on growth, sporulation and germination of Beauveria bassiana (Balsamo) Vuillemin. J. Entomol. Res. 32, 225-228. [ Links ]
Pava C., I. 2011. Degradación del suelo: problemática mundial y local. Centro de Estudios Políticos y Socioculturales del Caribe (CESPCA), Bogota. [ Links ]
Peña-Betancourt, S.D. and V. Conde-Martínez. 2012. Contenido de aflatoxinas y proteína en 13 variedades de fríjol (Phaseolus vulgaris L.). Rev. Mex. Cienc. Agric. 3, 201-206. [ Links ]
Quintero D., J.C. 2011. Degradación de plaguicidas mediante hongos de la pudrición blanca de la madera. Rev. Fac. Nal. Agr. Medellin 64, 5867-5882. [ Links ]
Reyes, Y., D. Infante, J. García-Borrego, E. Del Pozo, A. Cruz, and B. Martínez. 2012. Compatibilidad de Trichoderma asperellum Samuels con herbicidas de mayor uso en el cultivo del arroz. Rev. ProteciónVeg. 27, 45-53. [ Links ]
Robles-González, I.V., E. Ríos-Leal, J. Galíndez-Mayer, S. Caffarel-Méndez, J. Barrera-Cortés, F. Esparza-García, and H.M. Poggi-Varaldo. 2006. Comportamiento adsortivo-desortivo del lindano en un suelo agrícola. Interciencia 31, 305-308. [ Links ]
Rodríguez T., I.V., H. Morales, and C. Cardona M. 2003. Líneas base, dosis diagnóstico y medición periódica de resistencia a insecticidas en poblaciones de adultos e inmaduros de Trialeurodes vaporariorum (Homoptera: Aleyrodidae) en el Valle del Cauca, Colombia. Rev. Colomb. Entomol. 29, 21-27. [ Links ]
Rojan, P.J., R.D. Tyagia, D. Prévost, S.K. Brar, S. Pouleur, and R.Y. Surampallic. 2010. Mycoparasitic Trichoderm aviride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 29, 1452-1459. Doi: 10.1016/j.cropro.2010.08.004 [ Links ]
Roubos, C.R., C. Rodriguez-Saona, and R. Isaacs. 2014. Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol. Control 75, 28-38. Doi: 10.1016/j.biocontrol.2014.01.006 [ Links ]
Santoro, P.H., S.A. Cavaguchi, T.M. Alexandre, J. Zorzetti, and P.M.O.J. Neves. 2014. In vitro sensitivity of antagonistic Trichoderma atroviride to herbicides. Braz. Arch. Biol. Technol. 57, 238-243. Doi: 10.1590/S1516-89132014000200012 [ Links ]
Santos, A., E. Grijalba, M.V. Zuluaga. M. Gómez, and L. Villamizar. 2013. Compatibilidad in vitro de un bioplaguicida a base de Lecanicillium lecanii (Hypocreales: Clavicipitaceae) con agroquímicos empleados en los cultivos de algodón y berenjena. Rev. Colomb. Biotecnol. 15, 132-142. Doi: 10.15446/rev.colomb.biote.v15n2.38025 [ Links ]
Soto G., J.L., M. Andrade S., P. Mestas Z., M.M.O. Gonçalves, and H.H. Soto G. 2010. Impacto de herbicidas sobre microorganismos disolventes de fosfatos en suelo rizosférico de plantas de Solanum tuberosum. Teoría Praxis Inv. 5, 11-20. [ Links ]
Taj, A. and V.B.S. Kumar. 2012. In vitro interaction study of native Trichoderma harzianum isolates against Rhizoctonia solani, Colletotrichum gloeosporioides and Alternaria sp. of gerbera. Environ. Ecol. 30, 455-457. [ Links ]
Tofiño, A. 2000. Efecto de la mezcla de extractos de plantas ecoredudoq y algunos herbicidas sobre el comportamiento de las arvenses asociadas a los cultivos de caña de azúcar Saccharumsppcvs V5171 y CC 8592 y el híbrido de maíz SV 730 en el Centro del Valle del Cauca. Undergraduate thesis. Faculty of Agricultural Sciences, Universidad Nacional de Colombia, Palmira, Colombia. [ Links ]
Tofiño, A., D. Cabal, and L.F. Gil. 2012. Análisis de componentes del sistema productivo de aguacate, con incidencia probable de Phytophthora en Cesar, Colombia. Avanc. Inv. Agropec. 16, 63-90. [ Links ]
Tofiño R., A.P. 2013. Fisiología de cultivos del trópico seco: perspectivas de mejoramiento frente al cambio climático. Editorial Fondo de Publicaciones de la Universidad Popular del Cesar, Valledupar, Colombia. [ Links ]
Ulacio O., D., M. Jiménez T., W. Perdomo, and N. Moreno. 2012. Impacto de estrategias en la epidemiología de la pudrición basal, la pudrición carbonosa y el rendimiento de caraota (Phaseolus vulgaris L.). J. Selva Andina Res. Soc. 3,14-26. [ Links ]
UNL, Universidad Nacional del Litoral. 2010. Informe sobre grado de toxicidad del Glifosato. Expe No. 542212. Santa Fe, Argentina. [ Links ]
Xie, J., L. Hu, J. Tang, X. Wu, N. Li, Y. Yuan, H. Yang, J. Zhang, S. Luo, and X. Chen. 2011. Ecological mechanisms underlying the sustainability of the agricultural heritage rice-fish coculture system. Proc. Natl. Acad. Sci. USA 108, 1381-1387. Doi: 10.1073/pnas.1111043108 [ Links ]
Yadav, S.K., A. Dave, A. Sarkar, H.B. Singh, and B.K. Sarma. 2013. Co-inoculated biopriming with Trichoderma, Pseudomonas and Rhizobium improves crop growth in Cicer arietinum and Phaseolus vulgaris. Int. J. Agr. Environ. Biotechnol. 6, 255-259. [ Links ]
Zain, N.M.M., R.B. Mohamad, K. Sijam, M.M. Morshed, and Y. Awang. 2013. Effect of selected herbicides in vitro and in soil on growth and development of soil fungi from oil palm plantation. Int. J. Agric. Biol. 15, 820-826. [ Links ]














