Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.33 no.3 Bogotá Sep./Dec. 2015
https://doi.org/10.15446/agron.colomb.v33n3.51896
Doi: 10.15446/agron.colomb.v33n3.51896
1 Faculty of Basic Sciences, Universidad el Bosque. Bogota (Colombia); Agricultural Research Group (Grupo de Investigaciones Agricolas in Spanish), Faculty of Agricultural Sciences, Universidad Pedagogica y Tecnologica de Colombia (UPTC). Tunja (Colombia). hbalaguera@unbosque.edu.co
2 Centro Jose Acevedo y Gomez, Universidad Nacional Abierta y a Distancia. Bogota (Colombia)
3 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogota (Colombia)
Received for publication: 15 July, 2015. Accepted for publication: 17 November, 2015.
ABSTRACT
Cape gooseberry fruits are highly perishable with a limited shelf-life. The objective of this study was to evaluate the effect of refrigeration on the postharvest behavior of 1-methylcyclopropene-treated cape gooseberry fruits with the calyx. A completely randomized design with six treatments was used. The treatments were three storage temperatures (2, 6 and 16°C [ambient temperature]) and the application or absence of 1-methylcyclopropene (1-MCP; 1 mL L-1). The fruits were stored for 35 days. The fruits without refrigeration lasted 21 days. During the 35 days of refrigerated storage, the fruits at 2°C with 1-MCP showed a significantly lower respiration rate, color index and total soluble solids content and a higher firmness value and total acidity. Storage at 6°C also generated a favorable effect on the postharvest preservation of cape gooseberry fruits.
Key words: keeping quality, cold g, ethylene, respiration rate, Andean region.
RESUMEN
Los frutos de uchuva son altamente perecederos con limitada vida útil. El objetivo de esta investigación fue evaluar el efecto de la refrigeración sobre el comportamiento poscosecha de frutos de uchuva con cáliz tratados con 1-metilciclopropeno. Se utilizó un diseño completamente al azar con seis tratamientos, correspondientes a tres temperaturas de almacenamiento (2, 6 y 16°C [ambiente]), y la aplicación o no de 1-metilciclopropeno (1-MCP; 1 mL L-1). Los frutos fueron almacenados por 35 d. Se encontró que los frutos sin refrigeración tuvieron una duración de 21 d. Durante los 35 días de almacenamiento refrigerado, los frutos a 2°C con 1-MCP presentaron significativamente menor tasa respiratoria, índice de color y sólidos solubles totales, mayor firmeza y acidez total titulable. El almacenamiento a un efecto favorable en la conservación poscosecha de los frutos de uchuva.
Palabras clave: capacidad de conservación, almacenamiento en frío, maduramiento, etileno, tasa de respiración, Andes.
Introduction
Novoa et al. (2006) mentioned that Colombia is the biggest producer of cape gooseberries (Physalis peruviana L.) in the world. The Colombian ecotype has excelled in the world market because of its characteristic sweet taste, aroma and shiny color (Galvis et al., 2005). In 2013, Colombia had a production of 12,873 t over an area of 880 ha, giving a yield of 14.6 t ha-1, with the Boyaca Department being the biggest producer, followed by the departments of Antioquia, Cundinamarca and Nariño (Agronet, 2015). The cape gooseberry is considered a "Superfruit" because of its functional and medicinal properties (Fischer et al., 2011). These fruits are appreciated for their organoleptic properties, they are commercialized at national markets and exported as well to countries such as the United States, Canada, Holland, Germany, Belgium, Luxembourg and France, among others (Garcíaet al., 2008; Fischer et al., 2011).
The cape gooseberry is a perishable fruit with a limited shelf-life that exhibits a climateric behavior (Novoa et al., 2006; Gutiérrez et al., 2008) with an increase in ethylene synthesis at the climaterium stage (Gutiérrez et al., 2008; Valdenegro et al., 2012). Therefore, the evaluation of different technologies such as refrigeration and 1-methylcyclopropene (1-MCP) is necessary in order to preserve fruit quality for a longer time (Balaguera-Lópezet al., 2014a). In this regard, losses in quality and quantity between the moment of harvest and consumption affect the profitability of horticultural production. Postharvest losses in tropical countries have been estimated to exceed 50% of production (Paliyath et al., 2008).
Ethylene has an important role in the maturation and senescence processes of agricultural products that, depending on the case, produce desirable (faster and more uniform maturation) or undesirable effects (accelerated maturation and senescence that reduces shelf-life) (Balaguera-Lópezet al., 2014a). Different studies have indicated that ethylene could accelerate certain processes during the maturation of cape gooseberry fruits such as softening, antioxidant activity, and color change, among others (Trinchero et al., 1999; Majumder and Mazumdar, 2002; Gutiérrez et al., 2008; Valdenegro et al., 2012). Serek et al. (2006) and Watkins (2006) mentioned that 1-MCP is highly effective in protecting many agricultural species from the action of ethylene. 1-MCP occupies ethylene receptors in the membrane and thus prevents the binding of ethylene with the receptor and its action (Serek et al., 2006). In cape gooseberry fruits, the application of 1-MCP has shown to reduce ethylene production and respiration rates (Gutiérrez et al., 2008; Valdenegro et al., 2012).
In the same way, storage at low temperatures is the most important option for decelerating fruit deterioration (Téllez et al., 2007). Refrigeration is used to reduce respiration rates, which preserves fruit quality and delays senescence because respiration reductions also reduce the fruit metabolic and enzymatic activity (Silva et al., 2013). Refrigerated preservation under optimal conditions allows for the reduction of qualitative and quantitative losses due to physiological disorders or diseases, delaying maturation and senescence and extending the shelf-life of horticultural products with suitable quality for fresh consumption or industrial use (Paull, 1999; Martínez-Jávega, 1997). The correct use of refrigeration is one of the most effective ways of maintaining fruit quality (Kader, 2008). However, many tropical and subtropical fruits are sensitive to low temperatures, which becomes evident through different alterations and marks on the skin, generally known as lesions or chilling injuries, which can cause a big loss in commercial quality (Martínez-Jávega, 1997). In the cape gooseberry, studies on storage have been conducted at temperatures of 12°C (Novoa et al., 2006), 7°C (Lanchero et al., 2007) and 1.5°C (Alvarado et al., 2004), in which the importance of refrigeration in the postharvest preservation of this species has been highlighted.
According to Fischer et al. (1997; 2014), cape gooseberry fruits are harvested with a calyx that protects them, acting as a natural package. During the growing and maturation stage, the calyx protects the fruit from radiation and phytosanitary problems, acting as well as a mechanical barrier. After harvest, the calyx gives protection to the fruit against mechanical and pathogenic damage and reduces ethylene production and weight loss, extending the postharvest life, which is significant in relation to fruits without calyx (Fischer et al., 1997; Balaguera-Lópezet al., 2014b). In Colombia and the United States, consumers prefer fruits without the calyx, while exporting to Europe and Canada requires the presence of the calyx. Given that the conditions and requirements set forth for exporting to Europe and the United States are different, the special conditions at the moment of harvest, postharvest handling and commercialization have to be taken into account for each of these two markets (Garcíaet al., 2008).
In light of the above, the objective of this study was to evaluate the effect of refrigeration on the postharvest behavior of 1-methylcyclopropene-treated cape gooseberry (P. peruviana) fruits with the calyx.
Materials and methods
Cape gooseberry fruits of the ecotype Colombia were harvested from a commercial plantation in the municipality of Ventaquemada, Boyaca-Colombia, located at 2,630 m a.s.l., with an ambient temperature of 12°C. Fruits of a homogeneous size and in good phytosanitary and quality conditions were harvested at the green-yellow maturity stage, with a color index (CI) = 2.37, total soluble solids (TSS) = 13.37 Brix degrees and total titratable acidity (TTA) = 2.78%.
A completely randomized design with six treatments was used. The treatments were three storage temperatures (2, 6°C and ambient temperature (16°C)) and the application or absence of 1-methylcyclopropene (1-MCP; 1 mL L-1). Each treatment had four repetitions. Each of the 24 experimental units was composed of 125 g of fruits with the calyx. 1-MCP (Rohm and Haas, Bogota) was weighted in a beaker, which was placed inside a hermetic 20L chamber together with the fruits and hot water (45-50°C) was injected using a septa. The dissolution of 1-MCP in hot water liberated the gaseous 1-MCP to the headspace. The fruits were exposed to the treatment for 12 h at ambient temperature. When all the treatments had been applied, all of the fruits were packed in thermoformed polyethylene terephthalate (PET) packages and stored for 35 d at the chosen temperatures. The relative humidity was 90±5%.
The respiration rate was calculated for approx. 100 g of fruit in a 2 L hermetic chamber using a CO2 infrared sensor and a Labquest data collection device (Vernier, Beaverton OR). The CO2 values were registered every 4 s for 5 min. These values were used to calculate the slope that corresponded to the respiration rate and, in order to convert the data to CO2 kg-1 h-1, the fruit weight and chamber volume were taken into account. The color was measured using the CIELab system parameters L*, a* and b* with a Minolta digital colorimeter (Konica Minolta, Hong Kong). These data were used to calculate the color index applying the following equation: CI = (1000 x a*)/(L* x b*). The firmness of the fruits (N) was determined using a Lloyd LS1 digital texture analyzer (Lloyd LS1, Bognor Regis, UK) with a 1 KN load cell, 3 mm cylindrical die and Nexygen Plus software. The weight loss (%) was calculated from a fruit sample of 100 g, with which the fresh weight was determined using a 0.001 g precision scale. The total soluble solids (TSS) were determined by measuring the Brix degrees using a Hanna digital refractometer (Hanna, Woonsocket, RI) with a range of 0 to 85% and 0.1°Brix precision. The total titratable acidity (TTA) was determined using a 916 Food Ti-Touch 120 automatic titrator (Metrohm, Herisau, Suiza).
For the extraction and quantification of carotenoids, approx. 1 g of pulp was weighed, then 5 mL of acetone were added and the ingredients were mixed in a vortex for 1 min, after which the mixture was centrifuged for 10 min at 4,000 rpm. Then the supernatant was poured into a 25 mL volumetric flask, after which the acetone again was added to the pellet and the components were mixed again in a vortex and subsequently centrifuged. This procedure was repeated three times. The obtained supernatant was brought to 25 mL using acetone and the absorbancy was determined using a spectrophotometer at 450 nm. The quantification was done using a calibration curve with different concentrations of b-carotene. The total carotenoids were expressed as mg b-carotene/g FW (fresh weight).
The obtained data underwent normality tests (Shapiro-Wilk test) and homogeneity of variance (Levene's test). Then an analysis of variance and Tukey's multiple comparison test (P≤0.05) were performed using the SAS V. 9.4 software.
Results and discussion
Respiration rate: the lowest respiration rate was obtained with the refrigeration at 2°C and application of 1-MCP. As the refrigeration temperature decreased, the respiration rate decreased. Even at 2°C, the respiration rate was close to zero until 26 days after harvest (dah), after which the respiration rate increased until 35 dah. Furthermore, the fruits left at the ambient temperature had a shorter post- harvest life of 21 d (Fig. 1). In line with this, the refrigerated storage of the cape gooseberry fruits at 7°C resulted in a longer shelf-life of more than 40 days (Garcíaet al., 2014). Similarly, Alvarado et al. (2004) subjected cape gooseberry fruits to a temperature of 1.5°C for 16 d. The results of this showed that the fruits extended their postharvest shelf- life because, during the cold treatment, the fruits had a very low metabolic activity, which notoriously delays the normal processes that lead to advance in maturity. Studies conducted by Parra-Coronado et al. (2008) on plums made it possible to determine that respiration is related to temperature, where low temperatures inhibit respiration; this agrees with the results obtained for the cape gooseberry fruits.
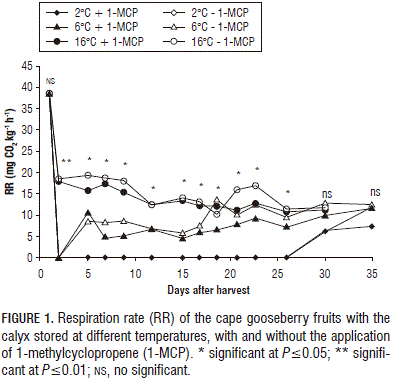
Barry and Giovannoni (2007) mentioned that ethylene has a close relationship with the respiration rate of fruits, which is why applications of 1-MCP are effective at reducing the respiration rate in different fruits, as has been reported for the tomato (Choi et al., 2008) and guava (Cerqueira et al., 2009), among others. The obtained results coincide with what has been reported for cape gooseberry fruits in previous studies, where the application of 1-MCP notably reduced the respiration rate (Gutiérrez et al., 2008; Valdenegro et al., 2012). Taking into account the fact that the respiration rate has a direct relationship with fruit perishability, refrigeration together with an application of 1-MCP could become a viable alternative for improving the preservation of cape gooseberry fruits with the calyx.
Skin color: The calyx had a CI value of -7.45 at the beginning of storage. This value became more negative with time in all the treatments, with the highest CI value observed at 21 dah in the fruits stored at 16°C without 1-MCP. At 35 dah, the fruits at 6°C without 1-MCP showed the highest calyx CI value (1.69), while the lowest calyx CI value during the entire storage was obtained in fruits refrigerated at 2°C+1-MCP with a value of -0.78 (Fig. 2A). In this same treatment, the lowest fruit skin CI value was obtained, where the CI value was always positive and continuously increased until the end of storage (Fig. 2B).
The results show that, during storage, the green color of the calyx diminished and developed a brownish color, just as it is reported by Balaguera-Lópezet al. (2014b), possibly due to chlorophyll degradation (Taiz and Zeiger, 2006). In this respect, Ligarreto et al. (2005) mentioned that, when the calyx matures, it gains a texture similar to parchment paper and becomes "straw-colored." Fischer et al. (2011) stated that the change in the calyx and fruit color is synchronic, which agrees with the results of this study because the general tendency in relation to the color change of the calyx and skin was similar (Fig. 2).
Color changes of cape gooseberry fruits are due to chlorophyll degradation and accumulation of carotenoids in plastids (Trinchero et al., 1999), mainly b-carotene (Fischer et al., 2000), and they are related to the presence of ethylene (Gutiérrez et al., 2008; Valdenegro et al., 2012). It is possible that the lower CI value in the fruits refrigerated at 2°C+1- MCP may have been due to a lower carotenoid accumulation as a result of a slower maturation process. Gutiérrez et al. (2008) reported that 1-MCP delays color change in cape gooseberry fruits, mainly in green-yellow fruits, which coincides with the results of this study. Several studies have shown that applications of 1-MCP reduce the chlorophyllase activity and loss of chlorophyll (Watkins, 2006; Sun et al., 2012). Tomatoes treated with 1-MCP showed a low lycopene accumulation and slower color change (Zhang et al., 2009). In addition, it is possible that refrigeration in cape gooseberry also may reduce the enzymatic activity related to color changes. In tomatoes, refrigerated storage was found to reduce the color change, expressed as Hue angle, which was due to a lower carotenoid accumulation (Rugkong et al., 2011).
Weight loss: A continuous increase in weight loss was observed, which was greater during the 1st d of storage, after which the loss rate became smaller, mainly under refrigeration conditions. The calyx can be considered a gas exchange organ, as has been reported by Kitagawa and Glucina (1984) for Japanese persimmon fruits (Diospyros kaki Thunb.). This is why, during the first days of storage, weight loss in fruits with the calyx depends more on the calyx than on the fruit, but, when the calyx is already dry, weight loss is almost entirely due to the fruit (Balaguera-Lópezet al., 2014b). Statistical differences were obtained at all samplings, except at 35 dah. A bigger weight loss was observed in fruits stored at the ambient temperature (11.65%), while a smaller weight loss was obtained with refrigeration at 6°C without 1-MCP (Fig. 3A).
At a lower temperature water, loss is smaller, which becomes evident through a low reduction in fresh weight and in appearance and quality properties, just as was found in this study. study. 1-MCP is able to reduce ethylene action (Serek et al., 2006) and, as a result, fruit degradation is slower and respiration and transpiration decrease. Therefore, the cape gooseberry fruits with 1-MCP and refrigeration (2 or 6°C) have a smaller chance of losing weight through respiration and transpiration, which in turn indicates that the fruits can exhibit a good postharvest behavior. Refrigeration also reduced weight loss in tomatoes (Javanmardi and Kubota, 2006) and banana passion fruits (Passiflora tripartita var. mollissima Bailey) (Téllezet al., 2007).
Chiabrando and Giacalone (2011) obtained similar results, with a smaller weight loss with the application of 1-MCP in blueberry fruits without generating undesirable changes in quality properties. Carrillo et al. (2011) found that 1-MCP (1 mL L-1) combined with storage at 7°C reduced weight loss in arazá (Eugenia stipitata Mc Vaug) fruits.
Firmness
A continuous reduction in firmness was observed. Significant differences were observed at all sampling points. The biggest softening occurred when the fruits were stored at the ambient temperature without 1-MCP. In turn, the fruits stored at 2°C and treated with 1-MCP showed the highest firmness value during the entire storage (Fig. 3B).
It is well known that one of the main causes of loss of firmness in fruits is the activity of enzymes that hydrolyze structural and reserve polysaccharides (Kays and Paull, 2004). In the cape gooseberry, the activity of polygalacturonases, pectin methylesterases and some glycosidases appears to be related to the loss of firmness in fruits (Majumder and Mazumdar, 2002). The depolymerization of pectins and xyloglucans has been found to be regulated by the presence of ethylene (Nishiyama et al., 2007; Pech et al., 2008). In the cape gooseberry, the PG enzyme (polygalacturonase) correlates with the presence of ethylene in fruits (Majumder and Mazumdar, 2002), which indicates the probable relationship that exists between the presence of ethylene and the loss of firmness in cape gooseberry fruits. In line with the abovementioned, tomatoes treated with 1-MCP were firmer and the polygalacturonase (PG) activity was suppressed (Choi and Huber, 2008). A smaller loss of firm- ness in cape gooseberry fruits as a result of the application of 1-MCP was also found by Gutiérrez et al. (2008).
Osterloh et al. (1996) stated that a high reduction in fruit firmness is due to a high metabolism and accelerated maturation derived from high temperatures. As a result, the genes related to the cell wall, PG (polygalacturonase), PE1 (pectin methylesterase 1), TBG4 (b-galactosidase 4), LeExp1 (expansin1), and XTH5 (xyloglucan endotrans-glucosilase/hydrolase 5) degradation in tomatoes were found to have a lower expression as a consequence of low temperatures (Rugkong et al., 2011). This shows the importance of refrigeration in maintaining firmness in cape gooseberry fruits.
Total carotenoids: Statistical differences were found at 20 dah in fruits with the calyx. Refrigeration at 2°C with and without applications of 1-MCP and refrigeration at 6°C with 1-MCP resulted in cape gooseberry fruits with the lowest total carotenoid content and the highest carotenoid content was obtained in the fruits stored at 16°C without 1-MCP. In turn, at 35 dah, the fruits with 1-MCP at 2 or 6°C showed the lowest concentration of total carotenoids (Fig. 4).
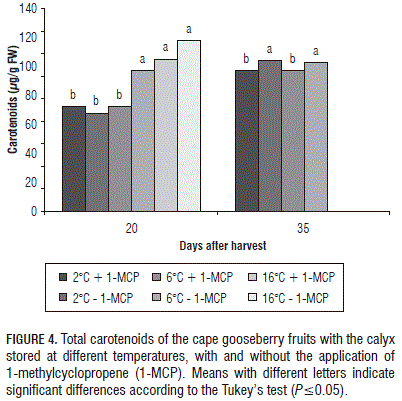
Refrigeration affects the reduction in carotenoid synthesis (Javanmardi and Kubota, 2006). In the tomato, refrigeration was found to decrease the synthesis of carotenoids, such as lycopenes and phytoenes, as a result of the lower expression of the genes PSY1 (phytoene synthase), CRTISO (carotenoid isomerase), GGPPS2 (geranylgeranyl pyrophosphate synthase 2), and DXS (xylulose 5-phosphate synthase) (Rugkong et al., 2011). Many of these enzymes are also involved in b-carotene synthesis, the main carotenoid in cape gooseberry fruits (Fischer et al., 2000), which would explain the reduction in synthesis of this carotenoid in the cape gooseberry.
Additionally, in oranges (Citrus sinensis L. Osbeck) var. Navelate, ethylene increased the total carotenoid content in the skin because it takes part in the expression of genes that codify for the synthesis of these pigments (Rodrigo and Zacarías, 2007). It is possible that carotenogenesis in cape gooseberry fruits also may be regulated by ethylene, which is why the application of 1-MCP reduced carotenoid synthesis. Similar results were observed in tomatoes, where 1-MCP significantly delayed lycopene accumulation (Choi et al., 2008). However, the highest reduction in carotenoid accumulation occurred when 1-MCP was combined with refrigeration at 2°C.
Total soluble solids: The TSS increased as a function of storage time. Statistical differences were found at 14 and 35 dah. At 21 dah, the fruits stored at the ambient temperature without 1-MCP showed the highest TSS content and, at 35 dah, the same was observed in fruits refrigerated at 6°C without 1-MCP. On the contrary, during the entire storage, the lowest TSS content was observed in the fruits with 1-MCP and stored at 2°C (Fig. 5A).
As fruits mature, part of the reserve polysaccharides are transformed into simple sugars such as glucose, fructose and sucrose, probably due to an increase in the activity of starch hydrolase enzymes (Kays and Paull, 2004; Paliyath et al., 2008), generating an increase in the TSS content. With refrigeration and 1-MCP, the increase in the TSS was lower because it was delayed by the maturation process. In pears, a lower increase in the total sugar content was also found using refrigeration and 1-MCP. The favorable effect of low temperatures on the TSS has been reported by Getinet et al. (2008) in the tomato and by Téllezet al. (2007) in banana passion fruits. Similarly, Marin et al. (2009) mentioned that 1-MCP can affect the metabolism of carbohydrates, just as was observed in this study. In turn, Singh and Pal (2008) found in "Safed" guava fruits that treatment with 1-MCP reduced the increase in the TSS. However, a greater response was expected with the application of 1-MCP and refrigeration, which may have been due to the fact that low temperatures can reduce the affinity or interaction of 1-MCP with ethylene receptors or maybe because the solubility of 1-MCP in the cytoplasm is reduced, as has been suggested by some studies (Mir et al., 2001; Villalobos et al., 2011).
Total tritatable acidity: The TTA diminished continu- ously. However, the general reduction was slower in fruits treated with 1-MCP and refrigerated at 2°C, while fruits without refrigeration and 1-MCP showed a greater reduction in the TA (Fig. 5B).
The organic acids are the second most important energy deposit for fruits, after carbohydrates (Osterloh et al., 1996). In this way, acidity is reduced because organic acids are respiration substrates (Kader, 2008) and/or are converted into sugars by means of gluconeogenesis (Paliyath et al., 2008). Taking into account the fact that refrigeration and the use of 1-MCP reduced the respiration rate in the cape gooseberry fruits (Fig. 1) and that Silva et al. (2013) mentioned that, with respiration reductions, both the fruit metabolic and enzymatic activity are reduced, it is possible to state that the reduction in the TA in this study was lower because the consumption of organic acids in respiration was probably lower. In this respect, Zhang et al. (2009) observed that 1-MCP delayed the reduction in the TA in tomatoes, while refrigeration did the same in curuba fruits (Téllezet al., 2007).
Conclusions
During the 35 d of storage, refrigeration at 2°C and the application of 1-MCP was the best preservation alternative for the cape gooseberry fruits with the calyx. With this treatment, the cape gooseberry fruits showed a lower respiratory rate, lesser loss of firmness, weight and total acidity, lower increase in total soluble solids, total carotenoids and color index in comparison with the control fruits. The storage at 6°C also enhanced the postharvest behavior of the cape gooseberry fruits.
Literature cited
Agronet. 2015. Producción nacional por producto, uchuva. In: www.agronet.gov.co; consulted: Jun, 2015. [ Links ]
Alvarado, P.A., C.A. Berdugo, and G. Fischer. 2004. Efecto de un tratamiento de frío (a 1,5°C) y la humedad relativa sobre las características físico-químicas de frutos de uchuva Physalis peruviana L. durante el posterior transporte y almacenamiento. Agron. Colomb. 22, 147-159. [ Links ]
Balaguera-López, H.E., F. Salamanca-Gutiérrez, J.C. Garcia, and A. Herrera. 2014a. Etileno y retardantes de la maduración en la poscosecha de productos agrícolas. Una revisión. Rev. Colomb. Cienc. Hortic. 8, 302-313. Doi: 10.17584/rcch.2014v8i2.3222 [ Links ]
Balaguera-López, H.E., C.A. Martínez, and A. Herrera. 2014b. Papel del cáliz en el comportamiento poscosecha de frutos de uchuva (Physalis peruviana L.) ecotipo Colombia. Rev. Colomb. Cienc. Hortic. 8, 181-191. Doi: 10.17584/rcch.2014v8i2.3212 [ Links ]
Barry, C.S. and J.J. Giovannoni. 2007. Ethylene and fruit ripening. J. Plant. Growth. Regul. 26, 143-159. Doi: 10.1007/s00344-007-9002-y [ Links ]
Carrillo, M.P., M.S. Hernández, J. Barrera, O. Martínez, and J.P. Fernández-Trujillo. 2011. 1-Methylcyclopropene delays arazá ripening and improves postharvest fruit quality. LWT - Food Sci. Technol. 44, 250-255. Doi: 10.1016/j.lwt.2010.05.029 [ Links ]
Cerqueira, T.S., A.P. Jacomino, F.F. Sasaki, and L. Amorim. 2009. Controle do amadurecimento de goiabas 'kumagai' tratadas com 1-metilciclopropeno. Rev. Bras. Frutic. 31, 687-692. Doi: 10.1590/S0100-29452009000300010 [ Links ]
Chiabrando, V. and G. Giacalone. 2011. Shelf-life extension of high- bush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chem. 126, 1812-1816. Doi: 10.1016/j.foodchem.2010.12.032 [ Links ]
Choi, S.T. and D.J. Huber. 2008. Influence of aqueous 1-methylcyclopropene concentration, immersion duration, and solution longevity on the postharvest ripening of breaker-turning tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 49, 147-154. Doi: 10.1016/j.postharvbio.2008.01.003 [ Links ]
Choi, S., P. Tsouvaltzis, C.II Lim, and D.J. Huber. 2008. Suppression of ripening and induction of asynchronous ripening in tomato and avocado fruits subjected to complete or partial exposure to aqueous solutions of 1-methylcyclopropene. Postharvest Biol. Technol. 48, 206-214. Doi: 10.1016/j.postharvbio.2007.10.008 [ Links ]
Fischer, G., P. Lüdders, and F. Torres C. 1997. Influencia de la separación del cáliz de la uchuva (Physalis peruviana L.) sobre el desarrollo del fruto. Rev. Comalfi 24, 3-16. [ Links ]
Fischer, G., G. Ebert, and P. Lüdders. 2000. Provitamin A carotenoids, organic acids and ascorbic acid content of cape gooseberry (Physalis peruviana L.) ecotypes grown at two tropical altitudes. Acta Hortic. 531, 263-267. Doi: 10.17660/ActaHortic.2000.531.43 [ Links ]
Fischer. 2011. Cape gooseberry (Physalis peruviana L.). pp. 374-396. In: Yahia, E.M. (ed.). stharvest biology and technology of tropical and subtropical fruits. Acai to citrus. Woodhead Publishing, Cambridge, UK. [ Links ]
Fischer, G., P.J. Almanza-Merchán, and D. Miranda. 2014. Impor- tancia y cultivo de la uchuva (Physalis peruviana L.). Rev. Bras. Frutic. 36, 1-15. Doi: 10.1590/0100-2945-441/13 [ Links ]
Galvis, J.A., G. Fischer, and O. Gordillo. 2005. Cosecha y poscosecha de la uchuva. pp. 165-190. In: Fischer, G., D. Miranda, W. Piedrahita, and J. Romero (eds.). Avances en cultivo, poscosecha y exportación de la uchuva (Physalis peruviana L.) en Colombia. Unibiblos, Universidad Nacional de Colombia, Bogota. [ Links ]
García B., H.R., A.C. Peña, and C. García. 2008. Manual de práctica de cosecha y acondicionamiento de la uchuva con fines de exportación. Corpoica; Ministerio de Agricultura y Desarrollo Rural (MADR), Bogota. [ Links ]
García, M.C., A.C. Peña, and B. Brito. 2014. Desarrollo tecnológico para el fortalecimiento del manejo poscosecha de la uchuva (Physalis peruviana L.). pp. 80-112. In: Carvalho, C.P. and D.A. Moreno (eds.). Physalis peruviana: fruta andina para el mundo. Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo (CYTED), Alicante, Spain. [ Links ]
Getinet, H., T. Seyoum, and K. Woldetsadik. 2008. Effect of cultivar, maturity stage and storage environment on quality of tomatoes. J. Food Eng. 87, 467-478. Doi: 10.1016/j.jfoodeng.2007.12.031 [ Links ]
Gutiérrez, M.S., G.D. Trinchero, A.M. Cerri, F. Vilella, and G.O. Sozzi. 2008. Different responses of goldenberry fruit treated at four maturity stages with the ethylene antagonist 1-meth- ylcyclopropene. Postharvest Biol. Technol. 48, 199-205. Doi: 10.1016/j.postharvbio.2007.10.003 [ Links ]
Javanmardi, J. and C. Kubota. 2006. Variation of lycopene, anti- oxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol. Technol. 41, 151-155. Doi: 10.1016/j.postharvbio.2006.03.008 [ Links ]
Kader, A.A. 2008. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 88, 1863-1868. Doi: 10.1002/jsfa.3293 [ Links ]
Kays, S.J. and R.E. Paull. 2004. Postharvest biology. Exon Press, Athens, GA. [ Links ]
Kitagawa, H. and P.G. Glucina. 1984. Persimmon culture in New Zealand. Sci. Inf. Publ. Cent., Wellington, New Zealand. [ Links ]
Lanchero, O., G. Velandia, G. Fischer, N.C. Varela, and H. García. 2007. Comportamiento de la uchuva (Physalis peruviana L.) en poscosecha bajo condiciones de atmósfera modificada activa. Corpoica Cienc. Tecnol. Agropecu. 8, 61-68. [ Links ]
Ligarreto, G., M. Lobo, and A. Correa. 2005. Recursos genéticos del género Physalis en Colombia. pp. 9-27. In: Fischer G., D. Miranda, W. Piedrahíta, and J. Romero (ed.). Avances en cultivo, poscosecha y exportación de la uchuva (Physalis peruviana L.) en Colombia. Unibiblos, Universidad Nacional de Colombia, Bogota. [ Links ]
Majumder, K. and B. Mazumdar. 2002. Changes of pectic substances in developing fruits of cape-gooseberry (Physalis peruviana L.) in relation to the enzyme activity and evolution of ethylene. Sci. Hortic. 96, 91-101. Doi: 10.1016/S0304-4238(02)00079-1 [ Links ]
Mir, N.A., E. Curell, N. Khan, M. Whitaker, and R.M. Beaudry. 2001. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of 'Redchief Delicious' apples. J. Amer. Soc. Hort. Sci. 126, 618-624. [ Links ]
Marin, A.B., A.E. Colonna, K. Kudo, E.M. Kupferman, and J.P. Mattheis. 2009. Measuring consumer response to "Gala" apples treated with 1-methylcyclopropene (1-MCP). Postharvest Biol. Technol. 51, 73-79. Doi: 10.1016/j.postharvbio.2008.06.008 [ Links ]
Martínez-Jávega, J.M. 1997. La frigoconservación en naranjas y mandarinas. Phytoma 90, 136-140. [ Links ]
Nishiyama, K., M. Guis, J.K.C. Rose, Y. Kubo, K.A. Bennett, L. Wangjin, K. Kato, K. Ushijima, R. Nakano, A. Inaba, M. Bouzayen, A. Latche, J.-C. Pech, and A.B. Bennett. 2007. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. J. Exp. Bot. 58, 1281-1290. Doi: 10.1093/jxb/erl283 [ Links ]
Novoa, R., M. Bojacá, A. Galvis, and G. Fischer. 2006. La madurez del fruto y el secado del cáliz influyen en el comportamiento poscosecha de la uchuva, almacenada a 12°C (Physalis peru- viana L.). Agron. Colomb. 24, 77-86. [ Links ]
Osterloh, A., G. Ebert, W.-H. Held, H. Schulz, and E. Urban. 1996. Handbuch der Lebensmitteltechnologie. Lagerung von Obst und Südfrüchten. Verlag Ulmer, Stuttgart, Germany. [ Links ]
Paliyath, G., D.P. Murr, A.K. Handa, and S. Lurie. 2008. Posthar- vest biology and technology of fruits, vegetables, and flowers. Wiley-Blackwell Publishing, New Delhi. [ Links ]
Parra-Coronado, A., J. Hernández-Hernández, and J. Camacho- Tamayo. 2008. Estudio fisiológico poscosecha y evaluación de la calidad de la ciruela variedad Horvin (Prunus domestica L.) bajo tres condiciones de almacenamiento refrigerado. Ing. Investig. 28, 99-104. [ Links ]
Paull, R. 1999. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 15, 263-277. Doi: 10.1016/S0925-5214(98)00090-8 [ Links ]
Pech, J.C., M. Bouzayen, and A. Latché. 2008. Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 175, 114-120. Doi: 10.1016/j.plantsci.2008.01.003 [ Links ]
Rodrigo, M.J. and L. Zacarias. 2007. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Technol. 43, 14-22. Doi: 10.1016/j.postharvbio.2006.07.008 [ Links ]
Rugkong, A., R. McQuinn, J.J. Giovannoni, J.K.C. Rosee, and C.B. Watkins. 2011. Expression of ripening-related genes in cold- stored tomato fruit. Postharvest Biol. Technol. 61, 1-14. Doi: 10.1016/j.postharvbio.2011.02.009 [ Links ]
Serek, M., E.J. Woltering, E.C. Sisler, S. Frello, and S. Sriskandarajah. 2006. Controlling ethylene responses in flowers at the receptor level. Biotechnol. Adv. 24, 368-381. Doi: 10.1016/j.biotechadv.2006.01.007 [ Links ]
Silva, D.F.P., M.R. Ribeiro, J.O.C. Silva, R.G.P. Matias, and C.H. Bruckner. 2013. Cold storage of peaches cv. Aurora grown in the Zona da Mata Mineira, Minas Gerais State, Brazil. Rev. Ceres 60, 833-841. Doi: 10.1590/S0034-737X2013000600012 [ Links ]
Singh, S.P. and R.K. Pal. 2008. Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharvest Biol. Technol. 47, 307-314. Doi: 10.1016/j.postharvbio.2007.08.010 [ Links ]
Sun, B., H. Yan, N. Liu, J. Wei, and Q. Wang. 2012. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and hinese kale. Food Chem. 131, 519-526. Doi: 10.1016/j.foodchem.2011.09.016 [ Links ]














