Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.34 no.1 Bogotá Jan./Apr. 2016
https://doi.org/10.15446/agron.colomb.v34n1.53074
Doi: 10.15446/agron.colomb.v34n1.53074
1 Tibaitata Research Center, Corporacion Colombiana de Investigacion Agropecuaria (Corpoica). Mosquera (Colombia). femartinez@corpoica.org.co
2 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogota (Colombia)
Received for publication: 16 September, 2015. Accepted for publication: 28 March, 2016.
ABSTRACT
This research sought to establish the response of the germination percentage (PG), synchrony index (E), mean germination time (MGT) and mean germination rate (MGR) of Annona squamosa L. seeds from Apulo (province of Cundinamarca) and Castilla (province of Tolima), Colombia, to treatments with 0, 50, 100, 200, 400, 600, or 800 mg L-1 of gibberellic acid (GA). All of the treatments with GA increased the PG at each point of time of seed incubation. The 600 mg L-1 GA treatment resulted in higher PGs (92.3% at 16 days for Apulo and 95% at 24 days for Castilla) and lower MGTs (8.75 and 5.38 days for Apulo and Castilla, respectively) than those found with the concentration of 0 mg L-1 GA (17.68 and 10.88 days for Apulo and Castilla, respectively). Also, treating the seeds with 600 mg L-1 GA generated higher MGRs (0.18 and 0.12 germinated seeds/day for Castilla and Apulo, respectively) than those obtained with 0 mg L-1 GA (Castilla = 0.09 and Apulo = 0.06 germinated seeds/ day). Likewise, the germination was synchronized with the application of any concentration of GA. The results evidenced a positive response to the GA application, which provided a tool for the characterization of the phenomenon of dormancy in the A. squamosa seeds.
Key words: germination percentage, mean germination rate, synchrony index, mean germination time.
RESUMEN
En esta investigación se buscó establecer la respuesta del porcentaje de germinación (PG), índice de sincronía (E), el tiempo medio de germinación (TMG) y la velocidad media de germinación (MGR) de semillas de anón Annona squamosa L. provenientes de Apulo (departamento de Cundinamarca) y Castilla (departamento de Tolima), Colombia, a tratamientos con 0, 50, 100 200, 400, 600 o 800 mg L-1 de ácido giberélico (GA). Todos los tratamientos con GA incrementaron los PG en cada punto del tiempo de incubación de las semillas. El tratamiento 600 mg L-1 GA generó mayores PG (92,3%, a los 16 días para Apulo y 95% a los 24 días para Castilla), menores TMG (8,75 y 5,38 días para Apulo y Castilla, respectivamente) que los hallados con la concentración de 0 mg L-1 GA (17,68 días para Apulo y 10,88 días para Castilla) y mayores VMG (0,18 y 0,12 semillas germinadas/día para Apulo y Castilla, respectivamente) que las obtenidas con 0 mg L-1 GA (Castilla= 0,09 y Apulo = 0,06 semillas germinadas/día). Así mismo, se observó una germinación más sincronizada con la aplicación de cualquier concentración de GA. Los resultados encontrados, evidencian la respuesta positiva a la aplicación de GA, lo cual brinda una herramienta en la caracterización del fenómeno de latencia en semillas de A. squamosa.
Palabras clave: porcentaje de germinación, velocidad media de germinación, índice de sincronía, tiempo medio de germinación.
Introduction
The sugar apple or anon (Annona squamosa L.) is the most widely distributed species of the Annona genus in the world. In Colombia, it grows on the Atlantic Coast and the dry areas of the valleys in the provinces of Valle, Cal- das, Huila, Tolima, Cundinamarca, Meta, and Santander, between 450 and 1,500 m a.s.l. (Buriticá and Cartagena, 2015). Although not yet officially exported, anon is a promising fruit, with high potential for international markets (Martínez, 2012).
The seeds of Annonaceae, within these A. squamosa, possess physical (impermeable seed coats) and morphological dormancy (Lemos et al., 1988; Adeniji et al., 2014; Baskin and Baskin, 2014). Morphological dormancy occurs when a rudimentary embryo occupies less than 30% of the seed volume at the time of seed dispersion, with an endosperm (endospermic seeds) that fills most of the seed and a differentiated embryo that needs specific conditions to complete its growth before germination (Baskin and Baskin, 2014). According to Pinto (2005), seed dormancy in Annonaceae is due to the degree of seed maturity and, among other factors, depends on climatic conditions during seed maturation on the mother plant and genetics of the mother plant. Therefore, difficulties in sexual propagation of the species of this family could be partially explained by seed dormancy.
In A. squamosa, the seeds feature a rudimentary but dis- tinctly underdeveloped embryo. According to Hayat (1963), the A. squamosa embryo is embedded in an abundant endosperm and has a slow growth rate, which could indicate the occurrence of morphological dormancy as specified by Baskin and Baskin (2004). The erratic and slow germination of these seeds has been reported by several authors, who proved that this can take around 30 d (Morton, 1987; Cruz, 2002), 50 d (Hernández, 1993) or 90 d (Hayat, 1963). Additionally, Stenzel et al. (2003) indicated that anon seeds have slow germination, with values that, after 2 months of germination, reach 1 to 3.8%.
Also anon seeds possess germination-inhibiting chemical substances that cause dormancy; these substances together with durable and waterproof integuments are antagonistic factors to rapid and uniform germination (Pawshe et al., 1997; Stenzel et al., 2003). The positive results of gibberellin (GA) treatments on the germination of A. squamosa has been reported by several authors (Pawshe et al., 1997; Stenzel et al., 2003; Sousa et al., 2005; Lima-Brito et al., 2006; Menegazzo et al., 2012) may also indicate the presence of morphophysiological dormancy in these seeds. Morphophysiological dormancy is characterized by an underdeveloped embryo and a physiological mechanism that inhibits seed germination (Baskin and Baskin, 2004; Baskin and Baskin, 2014). The embryo with this type of dormancy has a low potential or inhibition of growth due to a high ABA/GA ratio, which disables breaking through the seed cover structures (Baskin and Baskin, 2014).
Knowledge on seed germination is very important for the sexual propagation of anon as a cultivated species. At the same time, most germination studies for this species were conducted in regions with very differented edaphoclimatic characteristics to those prevailing in the producing areas of Colombia (Menegazzo et al., 2012; Adeniji et al., 2014). Also, there are contrasting reports on seed responses to the application of diverse hormones. Therefore, the aim of this study was to determine the effect of GA applications on the germination of A. squamosa seeds from two producing areas in Colombia.
Materials and methods
This research was conducted in the laboratories of Plant Physiology and Genetic Resources, Faculty of Agricultural Sciences, Universidad Nacional de Colombia, Bogota. The material (A. squamosa) was obtained from accessions collected from two locations: Apulo (province of Cundinamarca) and Castilla (province of Tolima) because plant individuals are distributed spontaneously in agroecosystems and not cultivated. This study was carried with fresh seeds obtained from ripe and soft-to-the-touch fruits, which were disinfected with 1% sodium hypochlorite immersion for 9 min, washed with distilled water and 96% ethanol three times, and, finally, rinsed three times with distilled water to remove any ethanol residue that may have remained on the seeds.
To assess the effect of GA treatments on germination, the seeds were soaked for 72 h in distilled water only (control, 0 mg L-1 GA) and GA at 6 different concentrations: 50, 100, 200, 400, 600, or 800 mg L-1 in closed glass jars. The treatments with control were arranged in a completely randomized design with five replicates per treatment. After soaking, the seeds were removed to assess the germination. The seeds were sown in disinfected peat substrate Klasmann® (Klasmann-Deilmann GmbH, Geeste, Germany) without nutrients at a depth equal to twice the seed length and subsequently placed in a controlled environment cabinet (SGC066, Sanyo Gallenkamp, Leicester, UK) for 30 d at a constant temperature of 35ºC in the absence of light because germination of seeds of A. squamosa is indifferent to light conditions (Ferreira et al., 2002). Observations were made every 5 d for 30 d, since seeds that did not germinate after 30 d could be classified as dormant (Baskin and Baskin, 2014). Germination was recorded in those seeds that had radicle protrusion.
With the sampling data, the following parameters were evaluated: germination percentage (GP) =  * 100, where, N = number of germinated seeds and Ns = total number of seeds; mean germination time (MGT) =
* 100, where, N = number of germinated seeds and Ns = total number of seeds; mean germination time (MGT) = 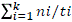 ; mean germination rate (MGR) =
; mean germination rate (MGR) = 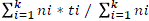 , where, ni = number of germinated seeds in the ith data collection; ti = time (in days) of the ith data collection and K = time (in days) duration of the germination test, and synchrony index (E) =
, where, ni = number of germinated seeds in the ith data collection; ti = time (in days) of the ith data collection and K = time (in days) duration of the germination test, and synchrony index (E) = 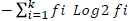 , where, ni = number of germinated seeds in the ith data collection and fi = relative frequency of germination (Ranal and Santana, 2006).
, where, ni = number of germinated seeds in the ith data collection and fi = relative frequency of germination (Ranal and Santana, 2006).
The assumptions of normality and homogeneity of variance were assessed using the SAS statistical package. The datas howed no residual normality and were transformed with arcosen function √(PG/100) usually used in germination studies (Júnior Wagner et al., 2006). ANOVA was performed and the differences among treatments were determined by the Tukey test (P≤0.05). SAS (Statistical Analysis System), version 9.1 was used.
PG response to the application of GA, for both areas was adjusted to a logistic model 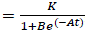 , where, K is the maximum value reached by the variable, in this case the seed weight (g) or the percentage of germination (%), A is the product of the initial value "a" maximum "K", B is a scale parameter on the time t that influences the growth rate. On the logistic model it was calculated an inflection point
, where, K is the maximum value reached by the variable, in this case the seed weight (g) or the percentage of germination (%), A is the product of the initial value "a" maximum "K", B is a scale parameter on the time t that influences the growth rate. On the logistic model it was calculated an inflection point 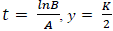 , in order to establish the change in the concavity of the curve in order to determine the growth dynamics of variation represented by the percentage of germination (Hsu, 1984).
, in order to establish the change in the concavity of the curve in order to determine the growth dynamics of variation represented by the percentage of germination (Hsu, 1984).
Results and discussion
The Fig. 1 shows the effect of GA application on PG of A. squamosa seeds obtained from Castilla, Tolima and its variation during 30 d of germination. Without the application of phytoregulator (0 mg L-1 GA) the germination was lower than the one achieved with any dose of the hormone; after 5 d of incubation 8.8% PG was obtained. According to the inflection point calculated for 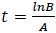 , during the first 8 d of incubation, the value of PG grew in geometric progression, that is, the PG presented high growth rates to reach half of the maximum value of its load (13.6%). After 8 d, the increases in the PG dwindled. The maximum PG of 27.2% was reached after 30 d.
, during the first 8 d of incubation, the value of PG grew in geometric progression, that is, the PG presented high growth rates to reach half of the maximum value of its load (13.6%). After 8 d, the increases in the PG dwindled. The maximum PG of 27.2% was reached after 30 d.
With the application of 200, 400, 600, and 800 mg L-1 GA, the better PG responses were observed during the incubation period. Under these concentrations, the germination patterns were similar, with values of PG much higher than found for the treatment of 0 mg L-1 GA in the first 5 d of incubation (86.4, 85.6, 89.6, and 84.8%, respectively). The shortest time to reach the maximum PG was obtained with the application of 600 mg L-1 at 16 d GA (PG = 92.3%), followed by 800 mg L-1 GA to 17.7 d (PG = 96.3%). The treatments of 200 and 400 mg L-1 GA obtained maximum PG values at 22 d of incubation (PG = 94.1%). For the same concentrations, geometric progression of PG or accelerated growth of this variable was presented during 1st d of incubation.
Without the application of phytoregulator (0 mg L-1 GA), the germination of the seeds from Apulo, was lower than those values achieved with any dose of the hormone. In this case, at 5 d of incubation PG was 9.6% and increased to a value of 59.3% at 30 d of evaluation. By analyzing the calculated inflection point, it could be observed that the accelerated growth of PG occurred until 18.9 d, where it reaches half the maximum load value (36.6%), after which the rate of PG growth slowed down (Fig. 2).
The seeds treated with GA at a concentration of 600 mg L-1 had the highest values of PG during the incubation time. The PG maximum of 95% was obtained with the shortest incubation time (between 24 and 28 d) compared to the other concentrations. The period of accelerated growth ended on 3.5 d of incubation and at 5 d of germination yielded a 56.8% PG. The curve reflects that the seed treatment with 800 mg L-1 GA presented a dynamics similar to that one found with treatment with 600 mg L-1 GA although the time to reach the maximum value of PG (88.4%) was obtained according to the projection model and parameters, after the last day of the incubation. The period of accelerated growth was 1.22 d and generated a PG of 56.8% during first 5 d of incubation.
There are few studies that describe patterns of germination in seeds of Annonaceae species. Stenzel et al. (2003) found that the seed germination of the cultivars Atemoya Gefner and Anon PR-3, when treated with 50-100 mg L-1 GA, occurred mainly between 14 and 28 d. Seed germination of cultivar PR-1 of Atemoya was presented mainly between 21 and 42 d when 50 mg L-1 GA was applied. Toll-Jubes et al. (1975) found a higher percentage of germination between 7 and 38 d in cherimoya (A. cherimola) seeds scarified and imbibed in a GA solution and water. Pawshe et al. (1997) found a higher percentage of germination performed at 22 and 26 d in seeds of A. squamosa immersed in GA at 50 and 100 mg L-1.
In the present study, there were observed differences in the patterns of seed germination of Castilla, Tolima and Apulo, Cundinamarca affected by the application of GA. At all GA concentrations, the seeds from Castilla, Tolima had higher PG values (> 70%) at 5 d of incubation than the seeds from Apulo (PG > 50%), and a shorter time to obtain the maximum germination value (maximum PG "K") and shorter periods (of PG accelerated growth, indicating that, in seeds from Tolima, stabilization occurred in less time.
GA effect on germination variables
In seeds from Apulo, only the concentration of 600 mg L-1 GA was effective in promoting a synchronized germination to generate a synchronization index significantly lower (0.34) than the control (0.41). In the seeds from Castilla, a trend was observed for a decreased synchrony index with increasing concentrations of GA. Again, it was possible to demonstrate differences in the response among the two locations, with the seeds of Tolima more influenced by the application of GA compared to the seeds of Cundinamarca (Fig. 3A).
The germination percentage (PG) was influenced by the external application of GA as could be seen in Fig. 3B. In both locations, all concentrations significantly increased the final PG (30 d incubation), as compared to the control (0 mg L-1). However, among the tested concentrations (50, 100, 200, 400, 600, and 800 mg L-1), statistically significant differences were not found. In seeds from Apulo, higher PG values were obtained in the treatments with 400 and 600 mg L-1 GA (96.8 and 97.6%, respectively), as compared to the control (59.2%), with an increase of 63 and 64.9% of PG. In the seeds from Castilla, one can see a trend in which the PG increased with increasing concentrations of GA. All concentrations contributed to a significant increase of PG (>200%) and, although these differences were minimal, the application of 800 mg L-1 GA resulted in higher values of PG (96%), which represented an increase of 252% over the control (PG = 27.2%).
Our results, obtained with Colombian assesions of A. squamosa, are comparable to those found by several authors in other tropical regions, where the PG increased after the application of GA at different concentrations. Pawshe et al. (1997), Stenzel et al. (2003), and Menegazzo et al. (2012) with low concentrations of GA (from 50 to 100 mg L-1) obtained the best PG (around 75%); the latter study reported durations of seed imbibitions in GA solutions from 5 to 24 has sufficient ones. Sousa et al. (2005) obtained 90% PG after imbibition of A. squamosa seeds for 12 hat 400 mg L-1 GA, indicating that doses of 50 to 750 mg L-1 GA also pro- duced positive results. Ferreira et al. (1998, 2002) reported that the application of 200 and 250 mg L-1 GA significantly promoted germination (up to 77%) of A. squamosa seeds in germination chamber at alternating temperatures of 20 to 30ºC. Likewise, Lima-Brito et al. (2006) found that the use of GA between 250 and 1000 mg L-1 increased the PG and IVG of the seeds.
In other Annonaceae species, the same positive effects were reported. De Smet et al. (1999) reported, in cherimoya seeds treated with 500; 1,000; 5,000, or 10,000 mg L-1 GA, a germination of 58.5, 65.5, 69.5, or 74.5%, respectively. Meanwhile, Gonzslez-Esquinca et al. (1997), indicated that seeds of A. diversifolia have dormancy that could be overcomed with the use of GA. Gimenez et al. (2014) in Annona emarginata obtained 91% germination with the use of 250 mg L-1 GA3. Ferreira et al. (2002) showed, in cultivars Gefner and Thompson of Atemoya, significant differences in germination between the concentrations 500, 750, and 1,000 mg L-1 GA (for cultivar Thompson) and 1,000 mg L-1 GA (for cultivar Gefner) compared with the control. At the same time, Stenzel et al. (2003) reported no differences between the control and GA concentrations (50 and 100 mg L-1) used for three cultivars of Atemoya (Gefner, PR-1, and PR-3).
Shorter germination times were recorded for treatments with GA for seeds from both locations, where a trend of a decreasing MGT with increasing concentrations of GA could be observed (Fig. 3C). In seeds from Cundinamarca, no statistical differences were found between the GA treatments during the time required for maximum germination (MGT), but the treatment with 600 mg L-1 GA gave the best values for MGT that decreased from 17.68 d (control data at 0 mg L-1 ) down to 8.75 d. In Castilla, Cundinamarca, the different GA concentrations were not statistically different in the time required for maximum germination (MGT); however, concentrations of 200, 400, 600, and 800 mgL-1 GA led to lower values of MGT of 5.52, 5.55, 5.38, and 5.82 d, respectively, compared to the control with MGT of 10.88 d.
Compared to the control (0 mg L-1 GA), the treatments of seeds of A. squamosa with GA at all concentrations were effective in promoting a greater MGR (Fig. 3D). It could be seen from the results for the two locations that the seeds tended to increase for the MGR with increasing concentrations from 0 to 600 mg L-1 GA. The concentration of 600 mg L-1 GA produced the highest MGR values for both seeds from Castilla, Tolima and Apulo, Cundinamarca (0.18 and 0.12 germinated seeds/d, respectively), compared with the values of the respective controls (Castilla = 0.09 and Apulo = 0.06 germinated seeds/d). However, the seeds from Castilla had significantly higher values of MGR relative to the control than the seeds from Apulo at each tested GA concentration, indicating a greater influence of the GA on the MGR of the seeds from Castilla, Tolima.
Increases in the speed of germination of the seeds of A. squamosa were also found with the application of 50 mg L-1 (Toll-Jubes et al., 1975), 100 mg L-1 (Stenzel et al., 2003) and 250 mg L-1 GA (Ferreira et al., 1999). Our results are also consistent with those of Sousa et al. (2008), who found that the highest rates of germination were obtained with GA treatments. Stenzel et al. (2003) in atemoya seeds indicated that GA increased the germination rate, where IVGs of 0.48 were obtained for cultivar Gefner and of 0.46 for cultivar PR-3.
The results showed a significant effect of the exogenous application of GA on seed germination, this being very effective treatment for breaking dormancy in seeds of A. squamosa. It is possible to observe a great sensitivity of the seeds to application of this phytoregulator since with any tested dose of GA, the responses were improved, indicating that the phenomenon of dormancy in these seeds might depend on a balance between hormonal promoters and inhibitors of growth (Weaver, 1987) and its breakage could be affected by the change in hormonal balance, in which GA acts to promote germination (Kigel and Galili, 1995).
With respect to the patterns of germination, GA treatments of seeds increased PG at each time point of incubation as the effect of large increases initial germination rates and shorter times to reach maximum PG, which was more pronounced in seeds from Tolima.
Although all of the GA concentrations, applied by soaking, enhanced the potential for seed germination, compared to the non-application of GA, it is possible to highlight the performance of the seeds treated with 600 mg L-1 GA. With this concentration, it took less time for the seeds to reach the maximum PG, also it generated a shorter time of accelerated growth and, therefore, the seeds had the highest PG in the first 5 d of incubation.
As mentioned, one of the characteristics of Annonaceae seeds is the presence of an immature, slow growing embryo that is not yet developed when the fruit is ripped. In this state, the embryo remains even after the seeds are removed from the fruits (Sanewski, 1991; De Smet et al., 1999). The positive response of the GA application on the seed germination was due to its effect on the embryo growth (Bewley, 1997). Additionally, the GA is required for the activation of embryonic growth and weakening of tissues (endosperm, perisperm, seed coats) surrounding the embryo and restricting its growth (Bewley, 1997; Bradford et al., 2000; Hegashi et al., 2002). During the germination, the embryo synthesizes and releases GA that promotes the production of various hydrolytic enzymes, including the hemicellulases, involved in the reserve solubilization of seeds. In seeds of Annonaceae, hemicellulases digest the reserves of galactomannans held in the endosperm (Baskin and Baskin, 2014), forming sugars that are further trans- ported to embryo growth, stimulating cell elongation and promoting the rupture of seed coats by the radicle, thus, improving greater uniformity of germination (Hopkins and Hüner, 2009). Likewise, many of the enzymes involved in the process of germination, such as lipases, proteases, phosphatases, and other hydrolases, are controlled or activated by plant regulators, such as GA (Arteca, 1995).
The difference between the seeds of two locations to treatment responses may be because plant responses to phytoregulators depend on many factors; among them are genetic and environmental ones that influence the level of endogenous hormones and substances antagonistic to germination (Agustí and Almela, 1991). Additionally, the depth of primary dormancy is highly related to the nutritional status of the mother plant (Geneve, 2003; El-Keblawy and Al-Rawai, 2006) and the environmental conditions during seed development (Finch-Savage and Leubner-Metzger, 2006); these conditions strongly influence the ABA/GA ratio in seeds at the final phases of seed maturation (Gutierrez et al., 2007) and this induced seed dormancy. The seeds from Apulo, Cundinamarca were collected at an altitude of 635 m a.s.l., an average temperature of 24.7ºC, a monthly average sunshine of 118.8 h and an annual rainfall of 1,146 mm, while seeds from Tolima were collected in a lower region, with an altitude of 447 m a.s.l., annual average temperature of 28.2ºC, average monthly sunshine of 183.23 h, and average annual rainfall of 1,732 mm. The habitat differences of the plant material could influence the seed responses of the same species to treat- ments (Nikolaeva, 2004).
Conclusions
The exogenous application of GA increased the unifor- mity of the germination and germination potential of the seeds of A. squamosa and generated large increases in the initial germination rates and germination rates during the incubation time.
There was agreat sensitivity of seeds to exogenously applied GA, indicating that the embryo may present low growth potential, which could explain the poor development and rudimentary characteristic.
Positive responses to the application of GA are valuable evidence that allows demonstrating a physiological component of dormancy in the seeds of A. squamosa.
Acknoledments
The authors express their gratitude to the Graduate School of the Faculty of Agricultural Sciences of the Universidad Nacional de Colombia, Bogota, for help in developing this Astudy.
Literature cited
Adeniji, I.T., A.F. Adio, O.A. Iroko, A.A. Kareem, O.C. Jegede, F. Kazeem-Ibrahim, T.O. Adewole, and A.O. Adeosun. 2014. Pre-treatment of seeds of Annona squamosa (sugar apple) a non timber forest product. Res. Plant Sci. 2, 50-52. [ Links ]
Agusti F., M. and V. Almela O. 1991. Aplicacion de fitorreguladores en citricultura. Aedos Editorial, Barcelona, Spain. [ Links ]
Arteca, R.N. 1995. Plant growth substances: principles and applications. Chapman & Hall, New York, NY. [ Links ]
Baskin, J.M. and C.C. Baskin. 2004. A classification system for seed dormancy. Seed Sci. Res. 14, 1-16. Doi: 10.1079/SSR2003150 [ Links ]
Baskin, C.C. and J.M. Baskin. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination. 2nd ed. Academic Press, San Diego, CA. [ Links ]
Bewley, J.D. 1997. Seed germination and dormancy. Plant Cell 9, 1055-1066. Doi: 10.1105/tpc.9.7.1055 [ Links ]
Bradford, K.J., F. Chen, M.B. Cooley, P. Dahal, B. Downie, K.K. Fukunaga, O.H. Gee, S. Gurusinghe, R.A. Mella, H. Nonogaki, C.T. Wu, H. Yang, and K.O. Yim. 2000. Gene expression prior to radicule emergence in imbibed tomato seeds. pp. 231-251. In: Black, M., K.J. Bradford, and J. Vazquez-Ramos. Seed biology: advances and applications. Proc. 6th Int. Workshop on Seeds, Merida, Mexico, 1999. CAB International, Wallingford, UK. [ Links ]
Buritica C., P. and J.R. Cartagena V. 2015. Neotropical and introduced fruits with special tastes and consistencies that are consumed in Colombia. Rev. Fac. Nal. Agr. 68, 7589-7618. Doi: 10.15446/rfnam.v68n2.50948 [ Links ]
Cruz P., E. 2002. Cultivo de anona. Boletín Técnico No. 7. Centro Nacional de Tecnología Agropecuaria y Forestal (Centa), San Salvador, El Salvador. [ Links ]
De Smet, S., P. Van Damme, X. Scheldeman, and J. Romero. 1999. Seed structure and germination of cherimoya (Annona cherimola Mill.). Acta Hortic. 497, 269-288. Doi: 10.17660/ActaHortic.1999.497.14 [ Links ]
El-Keblawy, A. and A. Al-Rawai. 2006. Effects of seed maturation time and dry storage on light and temperature requirements during germination in invasive Prosopis juliflora. Flora 201, 135-143. Doi: 10.1016/j.flora.2005.04.009 [ Links ]
Ferreira, G., C.P. Silva, E. Cereda, and J.F. Pedras. 1998. Efeito do ácido giberélico (GA3) na germinação de sementes de frutado-conde (Annona squamosa L.). pp. 186-187. In: Proc. 49 Cong. Nac. Bot. Sociedade de Botânica do Brasil, Salvador de Bahía, Brazil. [ Links ]
Ferreira, G.Z., L.A. Fogaça, and M.M. Malavasi. 1999. Germinación de semillas de Annona squamosa L., sometidas a diferentes tiempos y concentraciones de ácido giberélico. pp. 79. In: Mem. 2nd Cong. Int. Anonáceas. Universidad de Ciencias y Artes del Estado de Chiapas, Tuxtla Gutierrez, Mexico. [ Links ]
Ferreira, G., P.R. Erig, and E. Moro. 2002. Uso de ácido giberélico em sementes de fruta-do-conde (Annona squamosa L.) visando à produção de mudas em diferentes embalagens. Rev. Bras. Frutic. 24, 178-182. Doi: 10.1590/S0100-29452002000100039 [ Links ]
Finch-Savage, W.E. and G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Phytol. 171, 501-523. Doi: 10.1111/j.1469-8137.2006.01787.x [ Links ]
Geneve, R.L. 2003. Impact of temperature on seed dormancy. HortScience 38, 336-341. [ Links ]
Gimenez, J.I., G. Ferreira, and J.M. Corsato. 2014. Soluble sugars and germination of Annona emarginata (Schltdl.) H. Rainer seeds submitted to immersion in GA3 up to different water contents. Rev. Bras. Frutic. 36, 281-287. Doi: 10.1590/S0100-29452014000500033 [ Links ]
González-Esquinca, A.R., J.G. Álvarez M., and G.M. Porras P. 1997. Duración de la latencia e importancia de la cubierta dura y la inmadurez anatómica en la inbibición de la germinación de papausa blanca (Annona diversifolia Saff., Magnolidae, Annonaceae). Investigación, Ciencias y Artes en Chiapas (Unicach) 1, 37-46. [ Links ]
Gutierrez, L., O. Van Wuytswinkel, M. Castelain, and C. Bellini. 2007. Combined networks regulating seed maturation. Trends Plant Sci. 7, 294-300. Doi: 10.1016/j.tplants.2007.06.003 [ Links ]
Hayat, M.A. 1963. Morphology of seed germination and seedling in Annona squamosa. Bot. Gaz. 124, 360-362. Doi: 10.1086/336219 [ Links ]
Hegashi, E., R. Silveira, C. Golveia, and L. Basso. 2002. Ação fisiológica de hormônios vegetaisnas condições hídricas, metabolismo e nutrição mineral. pp. 139-158. In: Castro, P.R.C., J.O.A. Sena, and R.A. Kruge (eds.). Introdução à fisiología do desenvolvimiento vegetal. Editora da Universidade Estadual de Maringa (Eduem), Maringa, Brazil. [ Links ]
Hernandez, L.V. 1993. La reproducción sexual y reproducción vegetativa de las Anonaceas. Universidad Veracruzana, Xalapa, Mexico [ Links ]
Hopkins, W.G. and N.P.A. Hüner. 2009. Development: an overview. pp. 275-288. In: Hopkins, W.G. and N.P.A. Hüner (eds.). Introduction to plant physiology. 4th ed. John Wiley & Sons, Hoboken, NJ. [ Links ]
Hsu, F.H., C.J. Nelson, and W.S. Chow. 1984. A mathematical-model to utilize the logistic function in germination and seedling growth. J. Exp. Bot. 35, 1629-1640. Doi: 10.1093/jxb/35.11.1629 [ Links ]
Júnior Wagner, A., R.S. Alexandre, J.R.S. Negreiros, L.D. Pimentel, J.O.C. Silva, and C.H. Bruckner. 2006. Influência do substrato na germinação e desenvolvimento inicial de plantas de maracujazeiro amarelo (Passiflora edulis Sims f. flavicarpa Deg). Cienc. Agrotec. 30, 643-647. Doi: 10.1590/S1413-70542006000400008 [ Links ]
Kigel, J. and G. Galili. 1995. Seed development and germination. Marcel Dekker, New York, NY. [ Links ]
Lemos, E.E.P., R.L.R.R. Cavalcanti, A.A. Carrazoni, and T.M. Lobato. 1988. Germinação de sementes de pinha submetidas a tratamentos para quebra de dormência. pp. 675-678. In: Anais 9 Cong. Bras. Frutic. Campinas, Brazil. [ Links ]
Lima-Brito, A., V.C.A. Campos, J.R.F. Santana, and A.L.C. Dornelles. 2006. Efeito do ácido giberélico (GA3) na emergência de plântulas de Annona crassiflora Mart., Annona squamosa L. e Annona muricata L. Magistra 18, 27-33. [ Links ]
Martinez, F. 2012. Caracterizacion morfoanatomica de semillas de anon (Annona squamosa L.) y evaluacion de algunos parámetros fisiologicos del proceso de germinacion y latencia. MSc thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota. [ Links ]
Menegazzo, M.L., A.C. Oliveira, S.M. Kulczynski, and E.A. Silva. 2012. Efeitos de métodos de superação de dormência em sementes de pinha (Annona squamosa L.). Agrarian 5, 29-35. [ Links ]
Morton, J. 1987. Sugar apple (Annona squamosa). pp. 69-72. In: Morton, J. (ed.). Fruits of warm climates. Julia F. Morton Miami, FL. [ Links ]
Nikolaeva, M.G. 2004. On criteria to use in studies of seed evolution. Seed Sci. Res. 14, 315-320. Doi: 10.1079/SSR2004185 [ Links ]
Pawshe, Y.H., B.N. Patil, and L.P. Patil. 1997. Effect of pre-germination seed treatment on the germination and vigour of seedlings in custard apple (Annona squamosa L.). Ann. Plant Physiol. 2, 150-154. [ Links ]
Pinto, A.C.Q. 2005. Origin and distribution. pp. 17-20. In: Williams, J.T., R.W. Smith, A. Hughes, N. Haq, and C.R. Clements. (eds.). Annona species. International Centre for Underutilised Crops, University of Southampton, Southampton, UK. [ Links ]
Ranal, M.A. and D.G. Santana. 2006. How and why to measure the germination process? Rev. Bras. Bot. 29, 1-11. [ Links ]
Sanewski, E.T. 1991. Classification and cultivars. pp. 2-11. In: Sanewski, G. (ed.). Custard apples: cultivation and crop protection. 2nd ed. Queensland Department of Primary Industries, Brisbane, Australia. [ Links ]
Sousa, S.A. 2005. Cultura da Pinheira: caracterização de frutos, germinação e atributos de qualidade requeridos pelo sistema de comercialização. MSc thesis. Ciências Agrarias. Universidade Federal da Bahia, Salvador, Brazil. [ Links ]
Sousa, S.A., A.C.V.L. Dantas, C.R. Pelacani, E.L. Vieira, and C.A.S. Ledo. 2008. Superação da dormência em sementes de pinha. Rev. Caatinga 21, 118-121. [ Links ]
Stenzel, N.M.C., I.M. Murata, and C.S.V.J. Neves. 2003. Superação da dormência em sementes de atemóia e fruta-do-conde. Rev. Bras. Frutic. 25, 305-308. Doi: 10.1590/S0100-29452003000200031 [ Links ]
Toll-Jubes, J., H. Martinez, E. Padilla, and C.A. Oeste. 1975. Effects of mechanical scarification, substrate, seed position and gibberellic acid on germination of cherimoya. Rev. Agron. N.O. Argent. 12, 161-172. [ Links ]
Weaver, R.J. 1987. Reguladores del crecimiento de las plantas en la agricultura. 5th ed. Editorial Trillas, Mexico D.F. [ Links ]














