Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.34 no.2 Bogotá May/Aug. 2016
https://doi.org/10.15446/agron.colomb.v34n2.56799
Doi: http://dx.doi.org/10.15446/agron.colomb.v34n2.56799
Ecophysiological aspects of fruit crops in the era of climate change. A review
Aspectos de la ecofisiología de los frutales en los tiempos del cambio climático. Una revisión
Gerhard Fischer1, Fernando Ramírez2, and Fánor Casierra-Posada3
1 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogotá (Colombia). gfischer@unal.edu.co
2 Independent researcher. Bogotá (Colombia).
3 Faculty of Agricultural Sciences, Universidad Pedagogica y Tecnologica de Colombia. Tunja (Colombia).
Received for publication: 4 April, 2016. Accepted for publication: 30 June, 2016.
ABSTRACT
The increased concentration of carbon dioxide (CO2) and other greenhouse effect gases has led to global warming, which has resulted in climate change, increased levels of ultraviolet (UV) radiation and changes in the hydrological cycle, affecting the growth, development, production and quality of fruit crops, which undoubtedly will be difficult to predict and generalize because the physiological processes of plants are multidimensional. This review outlines how the effects of high/low solar radiation, temperature, water stress from droughts, flooding and rising levels of CO2 in the atmosphere affect fruit crops and their growth and physiology.
Key words: Radiation, temperature, water stress, carbon dioxide.
RESUMEN
La elevada concentración de dióxido de carbono (CO2) y otros gases de efecto invernadero ha resultado en un calentamiento global, mayores niveles de radiación ultravioleta (UV) y cambios en el ciclo hidrológico afectando el crecimiento, desarrollo, producción y calidad de los cultivos frutales, que sin duda, serán difíciles de predecir y generalizar debido a que los procesos fisiológicos de las plantas son multidimensionales. Se reseña, cómo los efectos de una alta y baja radiación solar y temperatura, estrés hídrico por sequía e inundación y el aumento del nivel de CO2 en la atmósfera inciden sobre los cultivos y afectan su crecimiento y fisiología.
Palabras clave: Radiación, temperatura, estrés hídrico, dióxido de carbono.
Introduction
Ecophysiology is the study of environmental effects on plant physiology; these conditions are of paramount importance for the success of any crop (Fischer and Orduz-Rodriguez, 2012). Ecophysiological research is conducted to describe the physiological mechanisms during development and growth of plants that interact with physical and biotic environmental factors (Lambers et al, 2008).
An orchard is characterized by an environment composed of light, temperature, water, humidity, wind, various atmospheric gases, soil nutrients and other conditions of the rhizosphere. During the growth of plants several climate and stress factors are influential at the same time for the crop, such as drought, heat, UV light, etc. (Mittler, 2006), i.e. no climatic factor alone can decide the physiological performance. For example, photosynthesis depends not only on radiation, but also on temperature, CO2, water and nutritional elements (Fischer and Orduz-Rodriguez, 2012). As shown by these authors, planting a crop in an eco-physiologically unfit place increases the costs of production and, thus, reduces the chance of high economic success. Taking into account environmental factors, at a given site, growing conditions decide on the size of the plant, the duration of phenological stages, the time and volume of the harvest.
Considering the dependence of crops on the environment, the effects of climate change can be very large depending on the fruit species and the climate conditions. It is very difficult to adopt experiences from one country to another one because the plant effect is different at each plantation site (Fischer and Orduz-Rodríguez, 2012). On the other hand, Pritchard and Amthor (2005) mentioned that it is difficult to predict and generalize how climate change would affect growth, development, production, and quality of crops since highly varied responses of plants resulting from physiological processes are multidimensional. In addition, climate change affects important plant pests with consequences on physiological potential, yield and quality of fruit species (Seidel, 2016).
Climate change, observed since 1950, has shown, among other factors, an increase in extremes of high air temperatures, reducing peaks of low temperature and an increase in the number of heavy rains in several regions. The average global temperature between the land surface and oceans shows an increasing trend of 0.85°C (0.65 to 1.06°C) for 1880-2012 (IPCC, 2015). For the 21st century, a temperature increase of nearly 3°C is projected by 2050 and values of as high as 6°C by the end of the century (Stöckle et al, 2011).
Pritchard and Amthor (2005) mentioned factors such as (a) the overall increase in the concentration of atmospheric carbon dioxide, (b) global warming related to the increase in the concentration of CO2 and other greenhouse gases, (c) increase in the concentration of ozone (O3) in the troposphere and the lower layer of stratospheric O3, (d) soil salinization in irrigated crops and (e) changes in the hydrological cycle as important phenomena of changing climate effects on agriculture. These authors claim that crops exposed to these conditions of altered growth must be able to harmonize the multiple effects of those changes to maintain a balance between the activities of the different organs.
To adapt fruit production to these new situations, mostly adverse to crops, a complete understanding of multiple effects of climate change on plant physiology is required (Swaminathan and Kesavan, 2012). The aim of this review was to elucidate how these factors, focusing on solar radiation, temperature, water and carbon dioxide, affect the physiology of the fruit plants, in general, with emphasis on fruits from the tropics and subtropics, and with some experiences of species from the temperate zones.
Effect of changing climatic factors on the ecophysiology of fruit plants
Solar radiation
The visible solar radiation is essential as a source of energy for photosynthetic activity in plants (Koyama et al., 2012), with its key role as an energy source for biomass production and finally fruit crops yield. If climate change causes high light intensity for long periods, the more CO2 is reduced and more carbohydrates produced that are available for filling and increasing the sweetness of fruits if soil moisture permits (Sherman and Beckman, 2003). In this context, an increased radiation and temperature require a lower leaf area in plants to produce the same amount of fruit as before climate change (Fischer et al., 2012a) and this situation gives space for varieties with higher photosynthetic performance and less susceptibility to early photoinhibition. The leaf area of a plantation is determined by the leaf area index (LAI), which is the ratio between the total leaf area and the floor area covered by it and can range in many fruit crops from 1.5 (minimum in apple) to 11 (maximum in citrus), depending on factors such as variety, rootstock, pruning, trellising, fertilization, and other cultural practices (Jackson, 1980; Fischer et al, 2012a).
As Casierra-Posada (2007) reported, reducing the leaf area may be a defense mechanism of plants to reduce the capture and use of light quanta. Thus, there may be two cases: a dynamic photoinhibition, which manifests itself during the midday hours, especially in the tropics, when the leaves are exposed to a large amount of incident radiation, and a chronic photoinhibition, which occurs as a result of failures or overload in foliar protection mechanisms (Casierra-Posada, 2007). The plants would be severely affected in its photosynthetic efficiency when photoinhibition results from alterations in radiation levels or temperature (Rivas, 2008).
A greater radiation absorption and reduced transpiration (stomatal closure) heat up the canopy, which affects the meristem temperature, without changing the surrounding air temperature, increasing or lowering the developmental rate of plants depending on the temperature range (Pritchard and Amthor, 2005).
Long periods of low radiation, with diffused or reduced light, stimulate the longitudinal growth of fragile vegetative structures because of an undersupply with carbohydrates (Dwivedi and Dwivedi, 2012). Therefore, the correct and not excessive density of plants and branches as well as the tree height and shape of the crown, which are regulated by pruning and espalier, and also by the direction of the rows from north to south, are important aspects to maximize light interception in plantations (Fischer and Orduz-Rodriguez, 2012). In banana passionfruit (Passiflora tripartita var. mollissima), in areas of high incidence of mist, the training and pruning of plant canopy in a 45° espalier, orientated to the east, increases interception of light and, therefore, fruit production (Miranda et al., 2009).
The reduction of light intensity affects the reproductive more than the vegetative phase because it directly influences floral induction, differentiation of flower bud, and also set, size, color, and organoleptic quality of fruits (Fischer et al., 2012b). Thus, in fleshy fruits, optimum solar radiation benefits color, synthesis of anthocyanin pigments, the refractive index (°Brix) and dry matter content, as well as increases the concentration of vitamin C (ascorbic acid) (Fischer and Orduz-Rodriguez, 2012; Fischer et al., 2016). A. Castro (personal communication, 2015) confirmed that, in the Colombian highlands within the orchard of deciduous fruit trees located at 2,500 m a.s.l. in Duitama (Boyaca), the high solar radiation produces apples of good coloration (through a high anthocyanin synthesis) and also with an increased thickness of the epidermis and cuticle, which are less susceptible to pathogens and insectpests.
However, during dry periods, longer than 15 days, such as the "El Niño" in the northern part of South America, plants can suffer from high solar radiation, when the chlorophylls in the thylakoid membranes of chloroplasts absorb excess light energy that can no longer be used in the photosynthetic process (Tadeo, 2000) causing photo-inhibition, which induces damage to the photosystem II (PS II) and degradation of D1 protein (Casierra-Posada, 2007). Moreover, high and prolonged solar radiation causes sunburn on juicy fruits and, through its additional effect on increasing the temperature in the irradiated cells, can generate fruit cracking (Fischer, 2000), an adverse effect that may be aggravated by pathogens attacking these unprotected tissues. In tomato plants, high light intensities caused a strong negative effect on the photosynthesis and leaf stomatal opening, reducing the [Ca2+]Cyt concentration from 252 to 52 nM in stomatal guard cells (O'Carrigan et al., 2014).
Climate change produces droughts (clear skies) and increases the levels of ultraviolet rays, especially UV-B (280-320 nm), that get higher with altitude and are getting worse because of the activities of man, which have led to the release of compounds that destroy the ozone layer (Martínez-Lüscher et al., 2014). To withstand the negative effect of increasing altitude, some fruit species, for example cape gooseberry (Physalis peruviana), have developed adaptations such as a dense pubescence that covers all of the green parts of the plant (Fischer and Melgarejo, 2014), but plant tolerance to moderate levels of UV radiation can be seriously affected by the progressive reduction of the ozone layer (Casierra-Posada, 2007). In general, crops should actively respond to environmental stress, such as O3 exposure, by increasing respiration rates of several repair and detoxification mechanisms, but these stand for a loss of assimilates that will no longer be used for increasing plant biomass (Pritchard and Amthor, 2005).
However, in sensitive plants, prolonged UV-B radiation can prevent photosynthetic activity and plant growth by damaging DNA, proteins, membranes, and lipids (Hideg et al., 2013). But, natural UV-B radiation levels can have favorable effects on several species, including the grapevine, favoring secondary metabolism, reducing abundant vegetative growth and the incidence of pathogens.
Plants are able to develop different protection mechanisms against UV-B radiation, such as an increased synthesis of phenylpropanoids (flavonoids e.g. anthocyanins) in the epidermis that absorb this radiation and act as antioxidants (Caldwell et al., 1998). M. Quijano (personal communication, 2012) informed that during berry ripening of vine grapes the UV light stimulates a higher synthesis of carotenoids, anthocyanins and flavonoids and, therefore, increases phenolic compounds that are important for im-provig taste, color and aroma of the wine. Das (2012) and Fischer and Melgarejo (2014) reported that UV-B radiation can increase leaf thickness and specific leaf weight, as a protective mechanism, at the expense of a reduced leaf area.
With low solar radiation, which occurs during rainy periods, fruits can be smaller due to reduced photosynthesis in the leaves close to them or have fewer grape berries per inflorescence on the vine or develop a poor color and brightness of its skin, such as in strawberries (Kays, 1999). If radiation levels fall below 10 to 30% of the light within the canopy, compared to those out of the canopy, the flowers are not differentiated in many fruit species (Rom, 1996) and production will occur only in the apical and lateral periphery of the tree (Sherman and Beckman, 2003).
Temperature
Das (2012) stated clearly that "plants can grow only within certain limits of temperature". Global warming stimulates crop growth and, thus, shortens the time of fruit formation, and the number of fruits and seeds within may be reduced by the effects of high temperatures on reproduction, particularly the formation and function of pollen (Larcher, 2003; Fischer and Orduz-Rodriguez, 2012). In peaches, a shortening of the earlier phases of fruit development by elevated temperatures can decrease fruit size and yield (Stöckle et al., 2011). These authors indicated that the shorter growing season would result in lower seasonal water loss by transpiration despite of increased temperature. Pritchard and Amthor (2005) estimated that an increase in air temperature by a few degrees will significantly reduce the yield of many crops, which are currently grown in typical producing regions and, moreover, extreme temperatures during anthesis, can severely affect harvest index. Countries in the northern hemisphere will benefit more from rising temperatures because the growing season will be extended (Kesavan and Swaminathan, 2012).
Advanced flowering, due to the increase in temperature, as reported by Ramírez and Kallarackal (2015, and the authors cited by them), occurs during several days, or even weeks, as compared to what happened 100 years ago, depending on the species. This reaction is more pronounced in the temperate zones than in the tropics or subtropics. Sherman and Beckman (2003) reported that peach cultivars with 80 days for fruit development, at an optimum temperature site, can take 120 days at a cooler place.
The temperature affects the rate of physiological processes, with great influence on the kinetic energy of the enzyme systems and each fruit species has an optimum temperature range, in the case of cape gooseberry between 13 and 18°C, in the Andean blackberry between 16-19°C, etc. (Fischer and Orduz-Rodriguez, 2012). The increase in average temperature can cause more flattened and less elongated fruits, especially when higher temperatures occur in the early phase of fruit development, when cell division takes place (Westwood, 1993).
The temperature increase in the era of climate change reduces the duration of phenological phases, as counted in "heat units" or "degree days" expressing the heat accumulation above a base temperature (Parra et al., 2015). In a recent study with pineapple guava (Acca sellowiana) in two growing zones with contrasting altitudes of Cundina-marca (Colombia), at 2,580 m and 1,800 m a.s.l., Parra et al. (2015) found the following base temperatures for four different reproductive phenological stages: (1) flower bud to anthesis 2.89°C, (2) anthesis to fruit set 3.04 °C, (3) fruit set to harvest 1.76°C, and (4) flower bud to harvest 1.74°C. In general, for citrus the physiological minimum is estimated at 12.5°C (Fischer and Orduz-Rodriguez, 2012), while in cape gooseberry Salazar et al. (2008) reported 6.29°C as a base temperature for stem growth.
High temperatures
High temperatures greatly affect fruit crops, especially with poor fruit set and decreases in production. For example, in grape vine, temperatures >35°C hinder fruit set, in cape gooseberry ≥30°C can inhibit flowering, in mango >35°C reduce the viability of pollen and fruit set (Fischer and Orduz-Rodriguez, 2012, and cited references therein). Hot tissues are softer and lose their texture and, hence, resistance to attacks by pathogens and insects-pests; in addition, high temperatures cause the degradation of organic acids required primarily for the respiration of ripe fleshy fruits and make them insipid (Fischer and Orduz-Rodriguez, 2012). Also, high night temperatures greatly degrade pho-toassimilates, affecting the filling and organoleptic quality of fruits (Das, 2012; Gariglio et al., 2007).
Global warming affects photosynthesis, especially in C3 plants, i.e. all commercially fruit species (except the few CAM fruit species); however, this effect has been little studied. In general, in C3 fruit plants, requiring lower temperatures, heat increases photorespiration because the Rubisco in C3 plants reacts with increased oxygenation to the cost of carboxylation and therefore a lower production of biomass than C4 plants (maize, sorghum, etc.) (Pritchard and Amthor, 2005). In their review about climate change on crop plants, Jarma et al. (2012) concluded that high temperatures can have adverse effects on physiological processes such as photosynthesis, respiration, water relations, hormone regulation and secondary plant metabolism, as well as on membrane stability.
Low temperatures
Climate change causes less events on extreme low temperatures in tropical and subtropical areas and, but in these areas, not enough chilling hours originate a shortage of low temperatures to break bud dormancy in deciduous fruits (Petri and Leite, 2004), such as apple, pear, peach, and plum. These species will demand higher concentrations of dormancy breaking products and varieties with lower requirements of chilling hours (Fischer, 2000). In addition, cool nights are necessary to reduce the maintenance respiration of fruits, which lowers their energy costs and increases the positive carbon balance and, hence, the accumulation of dry matter (Gariglio et al., 2007). Also, cool nights favor the coloring of fruits, with an increased production of anthocyanins (Sherman and Beckman, 2003). In wine grapes, cool nights advance berry coloration and, nowadays, indicate an important criterion for classifying grape-growing regions globally (Tonietto and Carbonneau, 2004).
In relation to the "El Niño" phenomenon, the fruit grower must not only avoid areas exposed to frost, but also has to take into account that crops such as pineapple, banana, starfruit, mango and papaya need climates with minimum temperatures of the coldest month of the year higher than 8°C (Paull and Duarte, 2011), also in the peach, night temperatures above 10°C force flowering (Sherman and Beckman, 2003).
Soil temperature
The soil temperature influences such important processes as the germination and emergence of seeds, absorption of water and nutrients and synthesis of hormones (cytokinins and gibberellins) in the roots, among others (Fischer and Orduz-Rodriguez, 2012). For example, in the citrus root zone, the temperature must exceed 12°C for bud sprouting and this event can be at any time of the year (Agusti, 2003).
Global warming will also increase soil temperature and, consequently, enhance soil organic matter decomposition, which may lead to soil fertility depletion (Osman, 2013), especially in hot dry o desert climates of the tropics. Too hot edaphic temperatures might harm the symbiosis between the roots and Rhizobia sp. and mycorrhizae (Pritchard and Amthor, 2005). As the optimum soil temperature for many tropical species lies between 20 and 25°C (Marschner, 2002), these should not exceed 30-32°C and above 35°C severely affects benefic soil microorganism (Fischer and Orduz-Rodriguez, 2012). Overheating of soils can be avoided by covers such as organic mulch and living short growing plants (e.g. short cut grass).
Water
Pritchard and Amthor (2005) reported an increase of 1 to 8% for the annual global precipitation, taking into account differences in their geographical distribution. In the past century, precipitation increased between 5 and 10%, preferably in areas of middle and high latitudes of the northern hemisphere, meanwhile, fell by 3% on average in the subtropical zone (Neenu et al, 2013).
Water not only plays a key role in plant physiological ecology but also in the enrichment of the planet atmosphere with oxygen. In the process of photosynthesis, two H2O molecules are broken to produce O2, released into the atmosphere, while the resulting hydrogen is used in the reduction of CO2 to carbohydrates (Taiz and Zeiger, 2010). In fruit trees, many juicy fruits contain between 80 and 90% water, while young twigs and leaves about 50-60% (Friedrich and Fischer, 2000).
Fruit are very demanding in water throughout plant reproductive stages starting from the flower formation until the filling of the fruit, considering that species with indeterminate growth, such as the Passifloraceae, Solanaceae and Caricaceae families, require a constant supply of water (Fischer et al., 2012b). In these species, water shortage stops growth and development, while heavy rains during flowering, fruit set or maturation are harmful for flowers and recently set fruits (Fischer and Orduz-Rodriguez, 2012).
Species with determinate growth (flowering, fruiting and harvesting occur in defined periods, as in citrus, mango, etc.) require about 1,000 to 2,000 mm annual rainfall, well distributed, especially from the start of the reproductive phase (Fischer and Orduz-Rodriguez, 2012). However, there is evidence that rainfall patterns, modified by climate change affect the phenology and reproductive behavior of many fruits, especially in the tropics (Ramírez and Kallarackal, 2015).
A prolonged rainy season or heavy rain after a long dry period can cause cracking of fleshy fruits, thus, water and nutrition have become of great interest to fruit growers (Fischer and Melgarejo, 2014). Fischer (2005) reported that an imbalance between the volume of water entering the fruit and extensibility of the epidermis and juicy fruits in the ripeness state are more susceptible to cracking by senescence of their epidermal layers.
Furthermore, high humidity environments inhibit transpiration which raises the pressure inside the fruit and therefore may cause cracking (Fischer and Melgarejo, 2014). Because of these reasons, the nutritional elements that influence the stability and extensibility of the skin play an important role in controlling this disorder (Fischer, 2005). Therefore, the soil in orchards must be kept at a constant moisture level, slightly below field capacity, with optimum contents of calcium, boron, potassium and magnesium, maintaining nitrogen fertilization at the low average levels (Gordillo et al., 2004; Fischer, 2005).
Water stress
Plant stress occurs whenever more water is lost through transpiration than absorbed from the soil (Kramer, 1989). Water is an important component of the cell's turgor pressure and essential media for biochemical processes; furthermore, a water deficit translates into dehydration, which severely affects the plant's metabolism and survival (Dwivedi and Dwivedi, 2012). Water stress is known to damage chloroplasts, thus, affecting photosynthesis (Kramer, 1989).
Early stomatal closure, effective cuticular transpiration, the ability to change leaf orientation toward the sun, or reduce leaf area (by abscission) are key aspects for cultivar selection for drought areas (Gariglio et al., 2007). Also, fruit tree cultivars with deep and expanded root systems are relevant during drought periods (Fischer and Orduz-Rodriguez, 2012).
Fruit trees have different mechanisms to overcome water stress. For example, leaves can extract water from fruits by mid-day stomatal closure (Westwood, 1993). Also, CAM (crassulacean acid metabolism) fruit plants such as cacti (Opuntia sp.) extract water from their fleshy cladoses through the phloem, under extreme water stress conditions (Fischer and Orduz-Rodríguez, 2012). Furthermore, prolonged water stress conditions during flowering and fruit filling in avocado are conducive to flower and fruit drop, which is a consequence of superficial growing roots (Paull and Duarte, 2011). In lulo (Solanum quitoense), fruit drop occurs if drought periods extend more than 3 weeks (Fischer and Orduz-Rodríguez, 2012).
Floral induction in Citrus sp. and some other subtropical fruit trees occurs in response to water stress conditions (Paull and Duarte, 2011). Similarly, floral induction in 'Arrayana' mandarin takes place in response to water stress conditions on oxisols in the foothills of Meta province, Colombia. In this zone, the "natural" water stress from late December through late February induces shoot initiation and flowering after a two week period (Orduz-Rodríguez and Fischer, 2007).
Water stress affects the number of fruits produced and their quality characteristics. Thus, fruits are smaller if water stress occurs during the cellular expansion phase (Gariglio et al., 2007). Fischer and Orduz-Rodriguez (2012) recommended, in fruit trees, in general, removing all plant parts that are unimportant for increasing productivity and fruit quality in prolonged "El Niño" scenarios. Plant parts to be removed include: basal and mature senescent leaves, unproductive branches, low quality fruits. Also, for the tree an adequate nutrient supply has to be guaranteed, such as potassium which reduces water consumption and phosphorous stimulating deep soil root growth.
Regulated deficit irrigation has been applied at selected phe-nological stages of fruit trees to control vegetative growth without yield reduction (Stöckle et al., 2011). Molina-Ochoa et al. (2015) found no yield or quality reduction in pear fruits in Sesquile (Cundinamarca, Colombia), when trees were irrigated with only 55% of the amount of water of the control plants.
Soil waterlogging
Large cropping areas within Colombia have been affected by climate change abiotic stresses such as waterlogging and flooding (Aldana et al., 2014). Both stresses have been intensified by climate change conditions. Since 2007, there has been an increase in heavy and prolonged rains in numerous provinces across Colombia. These heavy rains also can occur during the "dry" months of the year, affecting large scale fruit tree orchards lacking an efficient drainage system (Moreno and Fischer, 2014). Root anaerobic conditions are generated as a consequence of poor drainage (Das, 2012). Furthermore, many fruit trees require a water table level ≥1.5 m (Fischer and Orduz-Rodriguez, 2012).
In waterlogged soils, ionic buildup and anaerobe derived products generate phytotoxic conditions (Dwivedi and Dwivedi, 2012). These conditions increase the occurrence of fungal pathogens such as Phytophthora, Pythium and Fusarium (Villareal, 2014). Moreover, oxygen depletion inhibits water and nutrient uptake. And this in turn, reduces the stomatal resistance causing stomatal closure and negatively impacting photosynthesis (Moreno and Fischer, 2014).
Six to eight days of waterlogged conditions caused reduced plant biomass (particularly roots), flowering and fruit production in cape gooseberry. Furthermore, the plants died after 8 d in flooded conditions (Aldana et al., 2014). Moreover, cape gooseberry plants waterlogged over a six-day-period and inoculated with Fusarium oxysporum had a prominent reduction in root system, root width and foliar area; also, reduced photosynthesis and transpiration were evidenced by stomatal closure (Villareal, 2014).
Fischer and Orduz-Rodriguez (2012) reported that in plants sensitive to waterlogging fine and fibrous roots die first under hypoxic conditions, while leaves undergo chlorosis, due to deficient absorption and translocation of water and nutrients from the roots. As a consequence, leaves and fruits abscise and drop, respectively. This adversary effect increases with the rise in soil (and water) temperature within a climate change context.
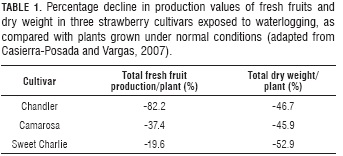
Casierra-Posada and Vargas (2007) found that waterlogging significantly affected the production of fruit and plant dry weight of strawberry cultivars, this situation reduced the production of fresh fruits in Chandler than in Sweet Charlie. In relation to the dry weight, the opposite occurred (Tab. 1), suggesting a differential tolerance among cultivars of this species to tolerate excess water in the soil.
Carbon dioxide
Because of the high level of emissions, the concentration of CO2 is now as high as 398 μmol mol-1 in the atmosphere (Swaminathan and Kesavan, 2012), the carbon dioxide level is one of the most limiting growth factor for fruit trees. Thus, the increasing CO2 concentration in the atmosphere will have a high impact on determining fruit tree productivity in the future since CO2 is a limiting factor for photosynthesis (Ramirez and Kallarackal, 2015). This is linked to the photosynthesis derived matter (85 to 92% of dry matter) (Larcher, 2003). In general, crops require from 150 to 220 kg ha-1 CO2 and this is supplied by the atmosphere though wind, air flow and turbulence (Fischer and Orduz-Rodriguez, 2012). Furthermore, it should be noted that publications pertaining fruit trees and elevated CO2 are relatively few in comparison to other crops (Ramirez and Kallarackal, 2015).
The increase of CO2 concentration in the air near the leaf blade decreases stomatal aperture, stomatal conductance and transpiration; in consequence, photosynthesis and growth increase because of an elevated water use efficiency (Pritchard and Amthor, 2005; Stöckle et al., 2011). An increase in growth because of an elevated CO2 requires higher water and fertilizer supply. This is because more nitrogen is required to ensure high crop productivity under climate change conditions (Ramirez and Kallarackal, 2015).
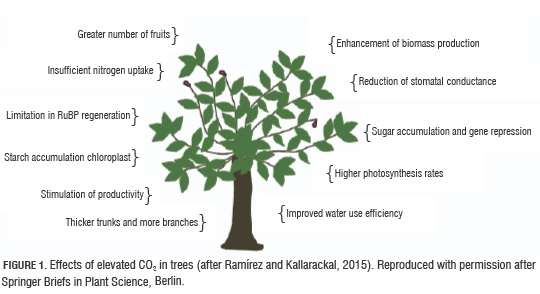
Hiratsuka et al. (2015) found that Satsuma mandarin increased gross photosynthetic rate of the fruit rind with increasing CO2 concentrations up to 500 μmol CO2 mol-1. Fischer et al. (2016) mentioned that an increase in atmospheric CO2 concentration improves the nutritional quality of fruits. Thus, Moretti et al. (2010, and the authors cited therein) reported that an elevated CO2 concentration had a positive effect on the postharvest quality of fruits and ascorbic acid increase in strawberries and oranges. Also, Bindi et al. (2001) observed that an elevated CO2 concentration increased total fenolics and flavonoids in grape, but, in mango, it decreased volatile compounds (Lalel et al, 2003). Bindi et al. (2001) found an increase in grape production, when the carbon dioxide concentration was shifted from 550 a 700 μmol mol-1 CO2, noting a 40 to 45% increase in production, respectively. Moreover, these authors reported no negative impacts on grape or wine quality. The effects of rising CO2 in plants are well known and include: reduced stomatal transpiration and conduction, increased water use efficiency, higher photosynthetic rates, augmented light use efficiency (Fig. 1) (Drake and González-Meler, 1997; Ramirez and Kallarackal, 2015). Whenever CO2 is increased from an ambient level of 350 to 550 ppm at 25°C, over time, the photosynthetic rates are reduced in some species relative to plants grown at ambient levels of CO2 (Ramirez and Kallarackal, 2015). This aspect is termed photosynthetic acclimation and has been attributed to five mechanisms that occur at the cellular level: (1) sugar buildup and gene repression (Krapp et al., 1993), (2) not enough nitrogen uptake by the plant (Stitt and Krapp, 1999), (3) a tie-up of carbohydrate accumulation with inorganic phosphate and a consequent limitation in RuBP renewal capacity (Sharkey, 1985), (4) starch buildup in the chloroplast (Lewis et al., 2002), and (5) triose phosphate consumption capability (Fig. 1) (Hogan et al., 1996). Furthermore, increased CO2 has been known to increase the following aspects in sour orange trees: truck diameter, the number of fruits produced and branch number (Fig. 1) (Kimball et al. (2007).
The fruit grower needs to find possible solutions to mediate the increase in CO2 concentration, thus, growers can increase nutrient and water applications and supply sufficient light for leaf growth and development (Ramirez and Kallarackal, 2015). Also, growers need to guarantee adequate "CO2 soil fertilization", which increases soil respiration; in Italy, organically managed vineyards (with manure and burying of pruning residues) showed higher soil respiration rates than conventional ones (Brunori et al., 2016). Applying organic fertilizers can increase the soil's CO2 production by 2/3 in the case of microorganisms and by 1/3 in the case of root respiration (Fischer and Orduz-Rodriguez, 2012).
Conclusions
Through its influence on the physiology of fruit plants, climate change affects differentially growth, development, production and quality of fruits that can be favorable in its response, but conversely if these factors occur at excessive levels.
Examples are solar radiation, which promotes photosynthesis, but in the case of too high levels can cause photoinhibition and/or sunburn. Increasing temperatures accelerate the crop cycle of the plant and enable crops at higher altitudes, but also increases the harmful effects of water stress and high radiation.
Elevated carbon dioxide will require growers to apply more nitrogen derived fertilizers and water. Growers need to mitigate climate change by selecting hardier cultivars that respond to elevated CO2 levels and are able to adapt to drought or waterlogged conditions.
The use of phenological scales (for example the BBCH [Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie] or the landmark stage proposed by Ramirez et al. (2014) and Ramirez and Davenport (2016) are another key factor for understanding the responses of trees to climate change.
Furthermore, there is an urgent need to develop mathematical models to be used in the particular case of fruit trees in the tropics. These models could help predict future scenarios and consequences of flooding, droughts, and elevated CO2, etc. in times of climate change.
Literature cited
Agusti, M. 2003. Citricultura. Ediciones Mundi-Prensa, Madrid. [ Links ]
Aldana, F., P.N. Garcia, and G. Fischer. 2014. Effect of waterlogging stress on the growth, development and symptomatology of cape gooseberry (Physalisperuviana L.) plants. Rev. Acad. Colomb. Cienc. Exact. Fis. Nat. 38, 393-400. Doi: 10.18257/raccefyn.114. [ Links ]
Bindi, M., L. Fibbi, and F. Miglietta. 2001. Free air CO2 Enrichment (FACE) of grapevine (Vitis vinifera L.): II. Growth and quality of grape and wine in response to elevated CO2 concentrations. Eur. J. Agron. 14, 145-155. Doi: 10.1016/S1161-0301(00)00093-9. [ Links ]
Brunori, E., R. Farina, and R. Biasi. 2016. Sustainable viticulture: The carbon-sink function of the vineyard agro-ecosystem. Agr. Ecosyst. Environ. 223, 10-21. Doi. 10.1016/j.agee.2016.02.012. [ Links ]
Caldwell, M.M., L.O. Bjõrn, J.F. Bornman, S.D. Flint, G. Kulandaivelu, A.H. Teramura, and M. Tevini. 1998. Effects if increased solar ultraviolet radiation on terrestrial ecosystems. J. Photochem. Photobiol. B: Biol. 46, 40-52. Doi: 10.1016/ S1011-1344(98)00184-5. [ Links ]
Casierra-Posada, F. 2007. Fotoinhibición: Respuesta fisiológica de los vegetales al estrés por exceso de luz. Rev. Colomb. Cienc. Hortic. 1, 114-123. Doi: 10.17584/rcch.2007v1i1.1150. [ Links ]
Casierra-Posada, F. and Y.A. Vargas. 2007. Crecimiento y producción de fruta en cultivares de fresa (Fragaria sp.) afectados por encharcamiento. Rev. Colomb. Cienc. Hortic. 1, 21-32. Doi: 10.17584/rcch.2007v1i1.1142. [ Links ]
Das, H.P. 2012. Agrometeorology in extreme events and natural disasters. BS Publikations, Hyderabad, India. [ Links ]
Drake, B.G. and M.A. González-Meler. 1997. More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 48, 609-639. Doi: 10.1146/annurev. arplant.48.1.609. [ Links ]
Dwivedi, P. and R.S. Dwivedi. 2012. Physiology of abiotic stress in plants. Agrobios, Jodhpur, India. [ Links ]
Fischer, G. 2000. Ecophysiological aspects of fruit growing in tropical highlands. Acta Hortic. 531, 91-98. Doi: 10.17660/ ActaHortic.2000.531.13. [ Links ]
Fischer, G. 2005. El problema del rajado del fruto de uchuva y su posible control. pp. 55-82. In: Fischer, G., D. Miranda, W. Piedrahita, and J. Romero (eds.). Avances en cultivo, poscosecha y exportación de la uchuva (Physalisperuviana L.) en Colombia. Unibiblos, Universidad Nacional de Colombia, Bogotá [ Links ].
Fischer, G., P.J. Almanza-Merchán, and F. Ramirez. 2012a. Source-sink relationships in fruit species. A review. Rev. Colomb. Cienc. Hortic. 6, 238-253. Doi: 10.17584/rcch.2012v6i2.1980. [ Links ]
Fischer, G. and L.M. Melgarejo. 2014. Ecofisiologia de la uchuva (Physalis peruviana L.). pp. 31-47. In: Carvalho, C.P. and D.A. Moreno (eds.). Physalis peruviana: fruta andina para el mundo. Programa Iberoamericano de Ciencia y Tecnologia para el Desarrollo - CYTED, Limencop SL, Alicante, Spain. [ Links ]
Fischer, G. and J.O. Orduz-Rodriguez. 2012. Ecofisiologia en frutales. pp. 54-72. In: Fischer, G. (ed.). Manual para el cultivo de frutales en el trópico. Produmedios, Bogotá [ Links ].
Fischer, G., A. Parra-Coronado, and D. Miranda. 2016. La calidad poscosecha de los frutos en respuesta a los factores climáticos en el cultivo. Agron. Colomb. Supl. 1. Doi: 10.15446/agron. colomb.sup.2016n1.58156. [ Links ]
Fischer, G., F. Ramirez y P.J. Almanza-Merchán. 2012b. Inducción floral, floración y desarrollo del fruto. pp. 120-140. In: Fischer, G. (ed.). Manual para el cultivo de frutales en el trópico. Produmedios, Bogotá [ Links ].
Friedrich, G. and M. Fischer. 2000. Physiologische Grundlagen des Obstbaues. Verlag Ulmer, Stuttgart, Germany. [ Links ]
Gariglio, N.F., R.A. Pilatti, and M. Agusti. 2007. Requerimientos ecofisiológicos de los árboles frutales. pp. 41-82. In: Sozzi, G.O.(ed.). Árboles frutales. Ecofisiologia, cultivo y aprovechamiento. Editorial Facultad de Agronomia, Universidad de Buenos Aires, Buenos Aires. [ Links ]
Gordillo, O.P., G. Fischer, and R. Guerrero. 2004. Efecto del riego y de la fertilización sobre la incidencia del rajado en frutos de uchuva (Physalis peruviana L.) en la zona de Silvania (Cundinamarca). Agron. Colomb. 22, 53-62. [ Links ]
Hideg, E., M.A. Jansen, and A. Strid. 2013. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 18, 107-15. Doi: 10.1016/j.tplants.2012.09.003. [ Links ]
Hiratsuka, S., Suzuki, M., H. Nishimura, and K. Nada 2015. Fruit photosynthesis in Satsuma mandarin. Plant Sci. 241, 65-69. Doi: 10.1016/j.plantsci.2015.09.026. [ Links ]
Hogan, K.P., D. Whitehead, J. Kallarackal, J.G. Buwalda, J. Meekings, and G.N.D. Rogers. 1996. Photosynthetic activity of leaves of Pinus radiata and Nothofagus fusca after 1 year of growth at elevated CO2. Aust. J. Plant Physiol. 23, 623-630. Doi: 10.1071/PP9960623. [ Links ]
IPCC, Intergovernmental Panel on Climate Change. 2015. Climate change 2014 - Synthesis report - Summary for policymakers. Switzerland. [ Links ]
Jackson, J.E. 1980. Light interception and utilization by orchard systems. Hort. Rev. 2, 208-267. Doi: 10.1002/9781118060759.ch5. [ Links ]
Jarma, A., C.C. Ayala, and H. Araméndis. 2012. Efecto del cambio climático sobre la fisiologia de las plantas cultivadas: Una revisión. Rev. UDCA Act. & Div. Cient. 15, 63-76. [ Links ]
Kays, S. 1999. Preharvest factors affecting appearance. Postharvest Biol. Technol. 15, 233-247. Doi: 10.1016/S0925-5214(98)00088-X. [ Links ]
Kimball, B.A., S.B. Idso, S. Johnson, and M.C. Rillig. 2007. Seventeen years of carbon dioxide enrichment of sour orange trees: final results. Glob. Change Biol. 13, 2171-2183. Doi: 10.1111/j.1365-2486.2007.01430.x. [ Links ]
Koyama, K., H. Ikeda, P.R. Poudel, and N. Goto-Yamamoto. 2012. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochem. 78, 54-64. Doi: 10.1016/j.phytochem.2012.02.026. [ Links ]
Kramer, P.J. 1989. Relaciones hidricas de suelos y plantas. Ediciones Harla, Mexico DF. [ Links ]
Krapp, A., B. Hofmann, C. Schafer, R.L. La Morte, G.W. Wall, D.J. Hunsaker, G. Wechsung, F. Wechsung, and T. Kartschall. 1993. Regulation of the expression of rbcS and other pho-tosynthetic genes by carbohydrates: a mechanism for the 'sink' regulation of photosynthesis? Plant J. 3, 817-828. Doi: 10.1111/j.1365-313X.1993.00817.x. [ Links ]
Lalel, H.J.D., Z. Singh, and S.C. Tan. 2003. Distribution of aroma volatile compounds in different parts of mango fruit. J. Hort. Sci. Biotechnol. 78, 131-138. Doi: 10.1080/14620316.2003.11511595. [ Links ]
Lambers, H., F.S. Chapin III, F. Stuart, and T.L. Pons. 2008. Plant physiological ecology. Springer, New York, NY. Doi: 10.1007/978-0-387-78341-3. [ Links ]
Larcher, W. 2003. Physiological plant ecology. Springer-Verlag, Berlin. Doi: 10.1007/978-3-662-05214-3. [ Links ]
Lewis, J.D., X.Z. Wang, K.L. Griffin, and D.T. Tissue. 2002. Effects of age and ontogeny on photosynthetic responses of a determinate annual plant to elevated CO2 concentrations. Plant Cell Environ. 25, 359-368. Doi: 10.1046/j.0016-8025.2001.00815.x. [ Links ]
Marschner, H. 2002. Mineral nutrition of higher plants. 2nd ed. Elsevier Science, London. [ Links ]
Martinez-Lüscher, J., N. Torres, G. Hilbert, T. Richard, M. Sánchez-Diaz, S. Delrot, J. Aguirreolea, I. Pascual, and E. Gomès. 2014. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochem. 102, 106-114. Doi: 10.1016/j.phytochem.2014.03.014. [ Links ]
Miranda, D., G. Fischer, C. Carranza, S. Magnitskiy, F. Casierra, W. Piedrahita, and L.E. Flórez (eds.). 2009. Cultivo, poscosecha y comercialización de las pasifloráceas en Colombia: maracuyá, granadilla, gulupa y curuba. Sociedad Colombiana de Ciencias Horticolas, Bogotá [ Links ].
Mittler, R. 2006. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11, 15-19. Doi: 10.1016/j. tplants.2005.11.002. [ Links ]
Molina-Ochoa, M.J., J.E. Vélez-Sánchez, and A. Galindo-Egea. 2015. Resultados preliminares del efecto del riego deficitario durante el periodo de crecimiento rápido del fruto de pera (var. Triunfo de Viena) en la producción y calidad del fruto. Rev. Colomb. Cienc. Hort. 9, 38-45. Doi: 10.17584/rcch.2015v9i1.3744. [ Links ]
Moreno, A. and G. Fischer. 2014. Efectos del anegamiento en los frutales. Una revisión. Temas Agrarios 19, 108-125. [ Links ]
Moretti, C.L., L.M. Mattos, A.G. Calbo, and S.A. Sargent. 2010. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 43, 1824-1832. Doi: 10.1016/j.foodres.2009.10.013. [ Links ]
Neenu, S., A.K. Biswas, and A. Subba Rao. 2013. Impact of climatic factors on crop production - A review. Agric. Rev. 34, 97-106. [ Links ]
O'Carrigan, A., E. Hinde, N. Lu, X.-Q. Xu, H. Duan, G. Huang, M. Mak, B. Bellotti, and Z.-H. Chen. 2014. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 98, 65-73. Doi: 10.1016/j.envexpbot.2013.10.007. [ Links ]
Orduz-Rodriguez, J. and G. Fischer. 2007. Balance hidrico e influencia del estrés hidrico en la inducción y desarrollo floral de la mandarina 'Arrayana' en el piedemonte llanero de Colombia. Agron. Colomb. 25, 255-263. [ Links ]
Osman, K.T. 2013. Soils: Principles, properties and management. pp. 129-159. In: Osman, K.T. (ed.). Plant nutrients and soil fertility management. Springer Science + Business Media, Dordrecht, The Netherlands. Doi: 10.1007/978-94-007-5663-2_10. [ Links ]
Parra, A., G. Fischer, and B. Chaves. 2015. Tiempo térmico para estados fenológicos reproductivos de la feijoa (Acca sellowiana (O. Berg) Burret). Acta Biol. Colomb. 20, 167-177. [ Links ]
Paull, R.E. and O. Duarte. 2011. Tropical fruits. Vol 1. 2nd ed. Cabi, Wallingford, UK. [ Links ]
Petri, J.L. and G.B. Leite. 2004. Consequences of insufficient winter chilling on apple tree budbreak. Acta Hortic. 662, 53-60. Doi: 10.17660/ActaHortic.2004.662.4. [ Links ]
Pritchard, S.G. and J.S. Amthor. 2005. Crops and environmental change. Food Products Press, The Haworth Press, New York, NY. [ Links ]
Ramirez, F. and T.L. Davenport. 2016. The phenology of the capuli cherry [Prunus serótina subsp. capuli (Cav.) McVaugh] characterized by the BBCH scale, landmark stages and implications for urban forestry in Bogotá, Colombia. Urban For. Urban Green. 19, 202-211. Doi: 10.1016/j.ufug.2016.06.028. [ Links ]
Ramirez, F., G. Fischer, T.L. Davenport, J.C.A. Pinzón, and C. Ulrichs. 2014. Mango trees have no distinct phenology: the case of mangoes in the tropics. Sci. Hortic. 168, 258-266. Doi: 10.1016/j.scienta.2014.01.040. [ Links ]
Ramirez, F. and J. Kallarackal. 2015. Responses of fruit trees to global climate change. Springer Briefs in Plant Science. Springer International Publishing, New York, NY. [ Links ]
Rivas, J. 2008. La luz y el aparato fotosintético. pp. 165-189. In: Azcón-Bieto, J. and M. Talón (eds.). Fundamentos de la fisiologia vegetal. McGraw-Hill Interamericana de España, Madrid. [ Links ]
Rom, C.R. 1996. Environmental factors regulating growth: light, temperature, water, nutrition. pp. 11-29. En: Maib, K.M., P.K. Andrews, G.A. Lang, and K. Mullinix (eds.). Tree fruit physiology: growth and development Washington State Fruit Commission. Good Fruit Grower, Washington. [ Links ]
Salazar, M.R., J. W. Jones, B. Chaves, A. Cooman, and G. Fischer. 2008. Base temperature and simulation model for nodes appearance in cape gooseberry (Physalisperuviana L.). Rev. Bras. Frutic. 30, 862-867. Doi: 10.1590/S0100-29452008000400004. [ Links ]
Seidel, P. 2016. Impacts of extreme weather events o pests, damage caused by pests and plant protection measures - first evidence. J. Kulturplanzen 68(9), 253-269. Doi: 10.5073/JfK.2016.09.02. [ Links ]
Sharkey, T.D. 1985. O2-insensitive photosynthesis in C3 plants. Its occurrence and a possible explanation. Plant Physiol. 78, 71-75. Doi: 10.1104/pp.78.1.71. [ Links ]
Sherman, W.B. and T.G. Beckman. 2003. Climate adaptions in fruit crops. Acta Hortic. 622, 411-428. Doi: 10.17660/ActaHortic.2003.622.43. [ Links ]
Stitt, M. and A. Krapp. 1999. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 22, 583-621. Doi: 10.1046/j.1365-3040.1999.00386.x. [ Links ]
Stöckle, C.O., J. Marsal, and J.M. Villar. 2011. Impact of climate change on irrigated tree fruit production. Acta Hort. 889, 4152. Doi: 10.17660/ActaHortic.2011.889.2. [ Links ]
Swaminathan, M.S. and P.C. Kesavan. 2012. Agricultural research in an era of climate change. Agric. Res. 1, 3-11. Doi: 10.1007/s40003-011-0009-z. [ Links ]
Tadeo, F.R. 2000. Fisiologia de las plantas y el estrés. pp. 481-498. In: Azcón-Bieto, J. and M. Talón (eds.). Fundamentos de la fisiologia vegetal. McGraw-Hill Interamericana, Madrid. [ Links ]
Taiz, L. and E. Zeiger. 2010. Plant physiology. 5th ed. Sinauer Associates, Sunderland, MA. [ Links ]
Tonietto, J. and A. Carbonneau, A. 2004. A multicriteria climatic classification system for grape-growing regions worldwide. Agr. For. Meteor. 124, 81-97. Doi: 10.1016/j.agrformet.2003.06.001. [ Links ]
Villareal, A. 2014. Evaluación fisiológica de plantas de uchuva (Physalis peruviana L.), en la respuesta al estrés por anegamiento e infección de Fusarium oxysporum. MSc thesis. Facultad de Ciencias Agrarias, Universidad Nacional de Colombia, Bogotá [ Links ].
Westwood, M.N. 1993. Temperate-zone pomology: physiology and culture. 3rd ed. Timber Press, Portland, OR. [ Links ]