Introduction
Maize is one of the most important crops worldwide. Its productivity can be altered by a number of plagues, includ ing the fall armyworm, Spodoptera frugiperda (J.E. Smith). It feeds on all growth stages of maize but most frequently in the whorl of young plants up to 45 days old (Cruz et al., 1999). During severe infestations they may completely de stroy the shoot (ICA, 2003). Larvae also feed on the kernels and young panicles (ICA, 2003). In Colombia, this insect may cause losses in corn crops ranging from 13% to 60% (ICA, 2008). Cultural practices, biological and chemical methods have been developed to help control S. frugiperda (ICA, 2003). In order to reduce the harmful effects of this lepidopteran, chemical insecticides are frequently used, but usually their effectiveness is limited by the behavior of the larvae, which tend to remain inside the shoot, usu ally surrounded by their own excrement, which prevents the insecticides from reaching those (Murua et al., 2013). These control methods also pose a significant economic burden for growers, through an increase in costs, produc tion losses, deterioration of the final quality of their corn and the presence of insecticide residues in the final product (ICA, 2008).
The use of genetically modified (GM) crops with resistance to herbivorous insects is an alternative in the control of such plagues. There are several commercial GM maize events with genes such as c rylab, cry2ab, crylF, cry3Bbl, cry34abl and cry35ab1, which confer resistance to lepidopteran insects. Among these, it has been demonstrated that the gene crylF confers resistance to S. frugiperda (EPA 2004, Barbour et al., 2008a, Hardke et al., 20ll). Its efficacy in the field has been tested in countries such as Argentina and Brazil (Murüa et al., 20l3; Nais et al., 20l3).
Commercially available maize bearing the gene crylF includes HERCULEX® I, also known as maize TCl507. HERCULEX® I contains a gene construct with two expres sion cassettes (Barbour et al., 2008a). The first cassette, which confers insect resistance, carries the crylF gene from Bacillus thuringensis var. Aizawi. The construct includes the maize ubiquitin promoter (Christensen et al., l992) followed by crylF (Peyne et al., l993; Cardineau et al., 200l) and the transcriptional terminator 3'ORF25 from Agrobacterium tumefaciens (Barker et al., l983). The gene crylF codes for the CrylF protein, which is classified as a delta-endotoxin. Cry proteins act by selectively binding to specific receptors on the surface of the mid-gut in suscep tible insects. This leads to the formation of pores which alter the ion flow in the mid-gut, causing intestinal paralysis and death due to bacterial sepsis (Bravo et al., 2007). The second expression cassette contains the gene pat (Wohlleben et al., l988), which codes for phosphinothricin acetyltransfer-ase (Pat), together with the 35S cauliflower mosaic virus (CaMV35S) promoter (Odell et al., l985) and terminator (Mitsuhara et al., l996). Pat catalyzes the acetylation of phosphinothricin (which is the active ingredient in ammo nium glufosinate-based herbicides), making it inactive as an herbicide (Dröge et al., l992). This is how HERCULEX® I maize is protected from damage by herbivorous insects and is tolerant to phosphinothricin herbicides.
In Colombia, HERCULEX® I has been approved for use as animal feed (ICA, 2006) and for environmental release under controlled conditions (ICA, 20l2).
Given that the fall armyworm is an economically important pest in Colombia (ICA, 2008), the introduction of resistance genes in Colombian maize cultivars is of potential benefit. With this aim, FENALCE has developed a conventional breeding program in which HERCULEX® I and non-transgenic hybrid maize parental lines have been crossed.
From these crosses, three hybrids were obtained which potentially carry the genetic constructs of maize TC1507. Here, we present the molecular characterization of these hybrids in order to detect the presence and function of the constructs, given that the characterization of the construct is essential for the eventual regulatory approval of the crop. In order to do this, the two parental lines, denominated 81 (non-GM) and 28 (GM), and the hybrids 286 x 285, 288 x 287 and 289 x 290, were evaluated. The analysis includes the presence/absence of the transgenes of interest and the functional evaluation through RNA and protein expres sion. All assays were performed twice.
Additionally, a Freedom to Operate (FTO) analysis was carried out for maize TC1507 in Colombia, in order to establish if its use violates any intellectual property rights.
Materials and methods
Transgene introgression
The crosses for the introgression were developed between 2008 and 2014 in the fields of the National Cereal and Legu me Research Center (CENICEL) Maize Breeding Program, located in Buenavista, Quindío Province. HERCULEX® I maize was crossed with elite lines developed in the Breeding Program and then backcrosses were carried out, assisted by complementary field tests and immunostrip assays in order to recover the genome of the elite lines. When this was achieved, the elite lines with the introgressed transgenes were hybridized, emphasizing the best hybrid combinations of the breeding program.
Transgene detection
The presence/absence of the genetic elements from HER-CULEX® I was tested. Five seeds per hybrid line were germinated under greenhouse conditions and leaves were obtained to perform duplicate assays. Primers were desig ned using the sequence of the genetic construct reported in patent US 7449564, SEQ ID 25 (Barbour et al., 2008a). The primers (Table 1) amplify the following fragments: 3' region of the maize ubiquitin promoter and 5' region of the cry1F gene, cry1F gene sequence, CaMV35S promoter, and pat gene. The presence of these four elements was tested by PCR. When necessary, primers for the constitutive zein gene were used.
The extraction of DNA and RNA from plant material was done with a DNA/RNA/Protein extraction kit from Nor-gen Biotek Corp (50 mg tissue for extraction). Polymerase KAPA3G from Biosystems was used for PCR. Reactions were developed in a final volume of 25 µiL containing PCR buffer 1X (1.5 mM Mg, 0.2 mM each dNTP), 0.3 µiM primers, and 1 U/µiL polymerase. Amplification condi tions for each fragment were determined by temperature gradient PCR. For PAT, the final Mg concentration used was 0.2 mM.
PCR conditions were as follows: initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 20 s, annealing at specific annealing temperatures (Ta) for 15 s and elongation at 72°C for 30 s. A final elongation at 72 °C for 30 sec was included. The annealing temperatures for each primer pair were: UBI-CRY: 63°C, CRY 1F: 63°C, CaMV35S: 60.6°C, PAT: 68°C, ZP1: 67.5°C.
Amplification fragments were visualized by electrophore-sis in 2% agarose gels stained with ethidium bromide. In all assays PCR mix with no DNA was used as an absolute control and the non-GM parental variety as a negative control. DNA ladder 1Kb plus from Invitrogen was used as a molecular weight marker.
PCR products obtained from primer pairs UBY-CRY1F, CRY1F and PAT were sequenced by Macrogen Inc. follow ing standard sequencing protocols with two reactions for each PCR product. The resulting sequence was compared against the expected sequence using the EBI platform (http://www.ebi.ac.uk/Tools/psa/emboss_water/nucleotide.html .).
Expression of cry1F and pat
The expression of the genes cry1F and pat in plant tissue was tested using RT-PCR and immunological assays.
RT-PC
Concentration and quality of extracted RNA was evaluated by absorbance at 260/280 nm and 260/230 nm. Any possible traces of DNA were removed using a Thermo ScientificTM DNaseI RNA free kit. The reaction mix consisted of 1 µj,g RNA, 1 µal 10X reaction buffer (100 mM Tris-HCl, pH 7.5 at 25 °C, 25 mM MgCl2, 1 mM CaCl2), and 1 µal DNaseI enzyme (1U/µji), taken to a final volume of 10 µjl with DEPC treated water. Kit instructions were followed. To verify that no DNA was left, a PCR with zein specific primers was carried out. DNA from parental 28 was used as a positive control.
For cDNA synthesis, a Thermo ScientificTM First Strand cDNA synthesis kit was used. Each reaction contained 1 µj,g RNA and 1 µJ,L primers oligoDT (100 µJ,M). These were incubated at 65°C for 5 min and the following were added: 4 µaL 5X reaction buffer (250 mM Tris-HCl at pH 8.3, 250 mM KCl, 20 mM MgCl2, 50 mM DDT), 1 µaL RiboLock RNase inhibitor (20 U/µaL), 2 µaL dNTP mix (10 mM), and 2 µJ,L M-MuLV reverse transcriptase (20 U/µL). Reactions were carried out according to manufacturer's instructions. The cDNA obtained was used for PCR with the primers CRY1F and PAT.
Immunoassays
The functionality of the proteins coded by each of the expression cassettes was tested through ELISA and im munostrip assays.
For the cry1F gene, an Agdia® ELISA Bt-Cry1F kit (cata logue number PSP1031) was used. From each sample, 0.15 g of leaf tissue were macerated in 3 mL extraction buffer. As negative controls, wells with buffer only and tissue from the non-GM parental line were included. Kit-provided Cry1F protein was used as a positive control. The assay was carried out according to manufacturer's instructions. Results were obtained visually and by measuring absorbance at 650 nm.
For the pat gene, an Amar Immunodiagnostics PVT Ltd. ELISA PAT/Pat kit (catalogue number AID027) was used. The assay was done according to manufacturer's instruc tions. A well with buffer only was included, and also two wells with the kit-provided negative control. Additionally, an immunostrip assay was carried out using Agrastrip® LL Strip Test Seed & Leaf from Romer Labs®.
Freedom to Operate analysis
An initial approach to FTO analysis was made in order to establish if the HERCULEX® I technology is protected by patents in Colombia. Two international databases were ac cessed: The Lens (https://www.lens.org/lens) and Patents-cope (http://www.wipo.int/patentscope/en). Colombia's national database was also queried (http://www.sic.gov.co/ es/banco-patentes). These three databases are open-access.
The following search terms were used: TCl507, maize, crylf, pat, lepidopteran resistance, Dow Agroscience, Pio neer. All relevant documents found were retrieved and a literal evaluation of their claims (which state the subject of protection) was done. Those patents found in international databases were used to search for their homologs in the Co lombian database. All retrieved documents were considered in order to determine the protection status in the country.
Results and discussion
Endotoxin CrylF in HERCULEX® I maize has been proven as an effective control of fall armyworm in this crop in countries such as Brazil and Argentina (Murüa et al., 20l3; Nais et al., 20l3). Here, the gene constructs from HERCU-LEX® I were evaluated for their presence and functionality in three hybrids derived from non-GM and GM maize.
Transgene presence
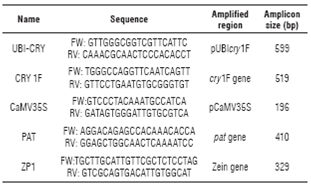
Primers UBI-CRY and CRY1F were used to detect the presence of the construct containing the gene cry1F. In the GM parental line 28 and the three hybrids, a 599 bp band was observed, which corresponds to part of the ubiquitin promoter and part of the cry1F gene (Fig. 1A, lanes 3-6). In the same plants, a 519 bp band was also detected, which is the predicted size for the amplified segment of the cry1F gene (Fig. 1B, lanes 3-6). As expected, no band was detected for the DNA extracted from the non-GM parental line (Fig. 1, lane 2). These results suggest that the maize lines where the PCR results were positive carry the Cry1F expression cassette in their genome.
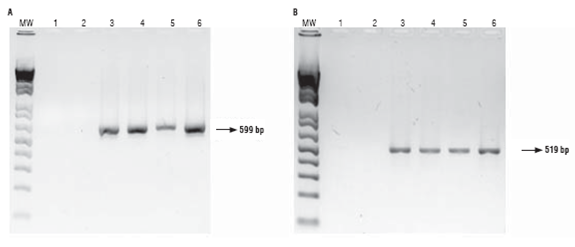
FIGURE 1 Gel electrophoresis of PCR products using primers UBI-CRY (A) and CRY1F (B). MW: molecular weight marker, 1: negative control (no DNA), 2: parental 81 (non-GM), 3: parental 28 (GM), 4: hybrid 286 x 285, 5: hybrid 288 x 287, 6: hybrid 289 x 290.
Primers CaMV35S and PAT were used to detect the pat gene expression cassette. As expected 196 bp and 412 bp bands were observed when using each primer pair, respectively, with DNA from the GM parental line 28 and the hybrids (Fig. 2, lanes 3-6), indicating that these plants have the genetic elements. No bands were observed for the non-GM parental line (Fig. 2, lane 2) as expected.
DNA), 2: parental 81 (non-GM), 3: parental 28 (GM), 4: hybrid 286 x 285, 5: hybrid 288 x 287, 6: hybrid 289 x 290.
Together, these results show that the GM parental line 28 and the three hybrids evaluated contain the transgenic ele ments from HERCULEX® I: cry1F with the ubiquitin pro moter and pat with the CaMV35S promoter. The ubiquitin promoter preceding cry1F is derived from an endogenous maize gene. For this reason, the primers designed cover part of the promoter and part of the gene to avoid obtain ing a signal from the endogenous gene and to detect the transgene only. For the pat expression cassette this was not necessary as both the promoter and gene are derived from bacterial sequences.
The sequences of the amplified fragments verified that they were in fact the HERCULEX® I elements. This analysis confirmed 577 bp for the amplicon from primers UBI-CRY, 467 bp for CRY1F and 377 bp for PAT.
Expression of genes cry1F and pat RT-PCR
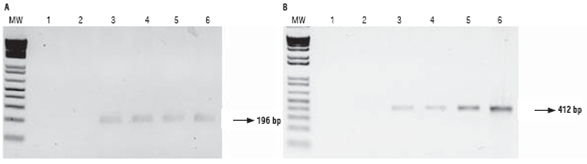
In order to establish if the constructs are functional in the hybrids, expression assays were performed. RNA was extracted from leaf tissue of the hybrids and visualized (Fig. 3A). The absence of DNA in the RNA samples after purification was confirmed by PCR using primers for zein (Fig. 3B); no bands were obtained for the RNA samples while the control (DNA from parental line 28) displayed a 329bp amplicon. This results show RNA extraction and purification was effective.
FIGURE 3 A. Gel electrophoresis of RNA extraction; B. Gel electrophoresis of RNA PCR products using primers ZP1. MW: molecular weight marker, 1: negative control, 2: parental 81 (non-GM), 3: parental 28 (GM), 4: hybrid 286 x 285, 5: hybrid 288 x 287, 6: hybrid 289 x 290, 7: positive control (parental 28 DNA).
Transcription of genes cry1F and pat was confirmed by RT-PCR using primers CRY1F and PAT, respectively. No band was obtained for samples from the non-GM parental line, while samples from the GM parental line and the hybrids produced a 519 bp band for cry1F and a 412 bp band for pat (Fig. 4).
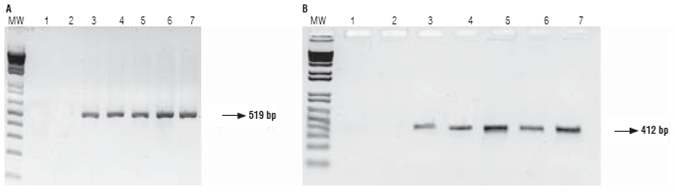
FIGURE 4 Gel electrophoresis of RT-PCR products using primers CRY1F (A) and PAT (B). MW: molecular weight marker, 1: negative control, 2: parental 81 (non-GM), 3: parental 28 (GM), 4: hybrid 286 x 285, 5: hybrid 288 x 287, 6: hybrid 289 x 290, 7: positive control (parental 28 DNA).
No color was observed for the samples of the non-GM parental line and negative control, while all samples from hybrid lines showed a distinct coloration similar to that of the positive control (Fig. 5A). These results were confirmed when absorbance was measured, as samples from hybrid lines had values at least three times higher than the nega tive control (0.165), which is the manufacturer's criterion to consider a result as positive (Fig. 5B). The non-GM sample had an absorbance value below that of the negativecontrol (0.055).
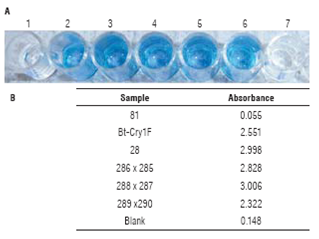
FIGURE 5 ELISA for Bt protein Cry1F. A. Visual results; 1: non-GM pa rental line 81, 2: Bt protein Cry1F, 3: GM parental line 28, 4: hybrid line 286 x 285, 5: hybrid line 288 x 287, 6: hybrid line 289 x 290, 7: blank; B. Absorbance values.
Detection of PAT/pat protein was also done by an ELISA with a PAT/pat specific antibody. When samples where PAT/Pat is present are added to the wells, these proteins bind the immobilized antibodies. These binding can be detected by a yellow coloration in the following step when the substrate is added and rinsed. Non-GM parental line 81 showed no color and had absorbance values below 0.06, similar to negative controls (Fig. 6). According to the manufacturer, the threshold to consider a sample as positive is an absorbance value equal to or greater than the mean absorbance for the negative control + 0.1, which in this case is 0.152. It is recommended to read absorbance using a primary 450 nm filter and a secondary 620/630 nm filter (for which its absorbance value is subtracted from the primary read). Absorbance values obtained for GM parental line 28 and the three tested hybrids are all above the threshold, indicating the presence of PAT protein.
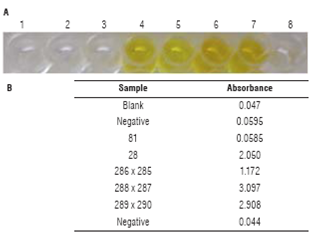
FIGURE 6 ELISA for PAT/Pat protein. A. Visual results; 1: blank, 2: ne gative control, 3: non-GM parental line 81, 4: GM parental line 28, 5: hybrid line 286 x 285, 6: hybrid line 288 x 287, 7: hybrid line 289 x 290, 8: negative control; B. Absorbance values
PAT protein was also detected using an immunostrip as say, where a specific antibody is linked to a color reactant on the lateral flow strip. When the strip is placed on a small volume of leaf tissue extract containing PAT, the protein binds to the antibody so a sandwich-like union is formed with the antibody bound to the color reactant. The membrane on the strip contains two capture areas, one for the linked PAT protein and another for the color reactant. These areas show a red color when the sandwich and/or the intact color reactant are captured. One visible red band (control band) indicates a negative sample, while two visible red bands indicate that a sample is positive for the presence of PAT. In accordance with the results of the ELISA, the immunostrip assay was negative for the non-GM parental line and positive for the GM parental line and the hybrids (Fig. 7).
Freedom to Operate analysis
Patents and patent-requests related to HERCULEX® I and the elements of the genetic construct were searched for. Two main patent families were found. The first family of patents derives from PCT request WO2004099447, which was filed by Pioneer Hi Bred International Inc., E.I. Du Pont de Nemours and Company, and Dow AgroSciences LLC. Derived from this request are United States patents US 7288643 B2 (Barbour et al, 2007), US 7417132 B2 (Barbour et al, 2008b), US 7435807 B1 (Barbour et al, 2008) and US 7449564 B2 (Barbour et al, 2008a). The first one protects a 11,361 bp sequence which includes the genetic construct and its flanking regions in the insert. Patents US 7288643 B2 and US 7435807 B1 protect two nucleotide sequences consisting of flanking regions which can be used to design primers for the specific detection of the event, and the amplicons produced from them. These patents have homo logs in Australia, Canada, South Korea, South Africa and Taiwan. Requests were also found for the European Union and Japan. Our search showed no requests for this patent in the Colombian national database.
A second patent family derives from PCT request WO2011075648, filed by Dow AgroSciences LLC. This request was also presented in Colombia under the file num ber 12 21231. It requests the protection of a method for the analysis of zygosity in maize event TC1507, not the protec tion of the event itself. This request entered the national phase on July 18th 2012. On March 17th 2014 the national authority issued a reply (record number 3447) stating that the claims do not achieve the required inventive level, after which the requesting company could file a reply. However, on August 5th 2014, the request was denied.
Databases were queried for patents related to genes cry1F and pat. For cry, the most relevant patents are in the fam ily "Protein Toxins Active Against Lepidopteran Pests", from Mycogen Corporation. The first in this family is US 5686069 (Payne et al., 1997), which protects the cry1F nucleotide sequence and genetic transformations where it is used. This patent request was filed in 1994, so it expired in 2014. No request for this patent was found in Colombia. Other patents related to cry1F are not directly associated with the subject of this study. Three requests were found in the Colombian database with file numbers 12119339, 12119367, and 12119370, which were denied 2014.
Patents related to gene pat are assigned to Bayer Crop Sci ence. The first are European patents EP 257542B1, in which the gene sequence and its use for plant transformation is protected, and EP 275957B1, which claims the codon-use optimization of the gene. Both these patents have already expired. In the United States, there is the family derived from patent US 5273894 (Strauch et al., 1993). None of these patents were requested in Colombia.
No patents related to promoters (CaMV35S or maize ubiquitin) or terminator sequences were found in Colombia.
Conclusions
Molecular evaluation of five maize lines (two parental lines and three hybrids) showed that the genomes of parental line 28 and hybrids 286 x 285, 288 x 287, and 289 x 290, carry the genetic elements characteristic of HERCULEX® I. The presence of the genes cry1F and pat, and their promoter se quences was detected by PCR. None of these elements were detected in parental line 81, which is non-GM, confirming the specificity of the assay. Sequencing of PCR products confirmed the identity of the amplified fragments. Tran scription of the expression cassettes for Cry1F and Pat in the GM parental line and the hybrids was demonstrated by RT-PCR, in contrast with the absence of this mRNA in the non-GM parental line 81. Immunological assays detected the expressed proteins Bt-Cry1F and Pat in the tissue of the GM parental line and the hybrids.
Together, these results demonstrate that the parental line 28 and hybrids 286 x 285, 288 x 287, and 289 x 290, have the genetic elements of HERCULEX® I thus they are function ally transcribed and translated into proteins Bt-Cry1F and Pat. As the assays presented here are qualitative, in order to establish if the genetic elements from HERCULEX® I are intact, further examination is necessary.
An initial approach to the FTO analysis indicates that the event HERCULEX® I was not patented in Colombia, this suggests that it could be used in the national territory with out detriment to the rights of third parties. These analyzes should be completed and updated, before considering any commercial use.