Introduction
The species Cavendishia bracteata and Thibaudia floribunda belong to the family Ericaceae, which, in the neotropics, is found in northeastern South America, mainly in Colombia, Ecuador, Peru, and Venezuela (Luteyn, 1983). In the Cundiboyacence plateau, the genus Cavendishia is distributed between 1,900 and 3,500 m a.s.l. and Thibaudia occupies areas between 2,300 and 3,600 m a.s.l. The species of these genera are frequently observed on exposed forest edges, roadsides, and roadside slopes (Luteyn, 1983; Vargas, 2002).
The Ericaceae plants in Colombia are represented by the highest number of species among the neotropics (Pedraza-Peñalosa et al., 2015) and distributed in the mountain regions, especially of paramo and subparamo, where they play an important ecological role in conservation of water resources (Luteyn and Pedraza-Peñalosa, 2008; Lagos-Burbano et al., 2010). The flowers produce nectar that feeds hummingbird pollinators and nectar robbers (Rojas-Nossa, 2013). In addition, many plants of this family have edible berries, which have been used for centuries as a source of food by the inhabitants of local and indigenous communities of the Andes region (Lagos-Burbano et al., 2010). Various native Andean Ericaceae have a potential as ornamental species or fruit crops, among which are plants of genera Vaccinium, Disterigma, and Macleania, with fruits that are consumed fresh or in cakes, jams, drinks (Luteyn, 1983; Echeverri and Lázaro, 2009; López et al., 2016) and used for ancestral medical practices (García, 1975; López et al., 2016).
Cavendishia bracteata, known locally as "uva de anís", is a shrub of 0.5-4 m height, with lanceolate coriaceous leaves of rounded base and acute apex (Toro and Venegas, 2002; Salinas and Betancur, 2005; Fig.1A and B); the newly emerged leaves have a bright red color. The inflorescences are grouped in short axillary or terminal clusters protected by a numerous oblong and pink bracts, with 6 to 9 whitish-red flowers. The flowers are pubescent, have tubular corolla typical of Ericaceae (Toro and Venegas, 2002; Salinas and Betancur, 2005). The fruit is a globose glabrous berry, 8 to 15 mm in diameter, is pubescent when green and pink to purple when ripen, have pleasant anise taste and contains about 100 seeds (Lagos-Burbano et al., 2010). Both leaves and fruits possess high levels of antioxidants (Plazas et al., 2015; Puertas-Mejía et al., 2015).

FIGURE 1 Plants (A) and fruits (B) of Cavendishia bracteata; plants (C) fruits (D) of Thibaudia floribunda.
Thibaudia floribunda or "uvo" is a terrestrial or epithytic shrub of 1 to 3 m height (Fig. 1C and D). Its large petiolated leaves reach 30 cm length, have oblong to ovate lamina, acute to acuminate apex and straight margin (Vargas, 2002). The pendulous inflorescences are terminal or axillar and grouped in clusters, with 10-20 tubular flowers of pink to red color; the pedicels are glabrous or frequently covered with thick pink to red hairs (Salinas and Betancur, 2005). The fruit is a berry, 4 to 6 mm in diameter, blankish-yellow when ripen and of slightly acid taste (Vargas, 2002); the leaves are known as a source of antioxidants (Plazas et al., 2015).
At present, there is insufficient information about propagation methods that could facilitate introducing these fruit trees from the wild into the crop production; equally it is unknown the potential agronomic use of these native Andean species, when exploring their opportunity to reache national and international markets. The propagation through cuttings allows reducing the time to flowering and fructification in fruit trees and shrubs. The use of growth regulators, such as the exogenous applications of auxins, has become one of the most popular practices to ensure rooting (Stuepp et al., 2017). These phytohormones stimulate root formation and growth (Baldini, 1992; Blakesley, 2013; Braha and Rama, 2016). Due to the initiation of primordia of adventitious roots affecting root growth (Druege et al., 2016).
Indole acetic acid (IAA) is the main auxin of plant origin, while indole butyric acid (IBA), naphthalene acetic acid (NAA), and indole propionic acid are often used in the practices of plants propagation (Ludwig-Müller and Cohen, 2002). Previous studies established the ranges in auxin concentrations useful for vegetative propagation of ericaceous species by cuttings. For rooting of native Andean Ericaceae, the application of auxins in concentrations of 400 mg L-1 NAA assured rooting of 44% for agracejo (Vaccinium floribundum) and of 77% for ovo (Disterigma alaternoides), with best results achieved on cuttings taken from the apical section of the branches (Magnitskiy et al., 2011). The benefit of auxin use for rooting of uva camarona (Macleania rupestris), where the treatments of 500 mg L-1 of IBA or NAA resulted in the maximum number of roots in apical or semi-hardwood cuttings (Veloza et al., 2014).
It is necessary to evaluate the effect of different concentrations of auxins (NAA) in other undomesticated fruit species and, in turn, obtain the bases for the protocol that allows the use of potential fruit crops, such as T. floribunda and C. bracteata, in efficient and practical way. In the present study, the origin of cuttings (apical and basal) and the concentration of NAA were evaluated as factors that could influence the rooting process.
Materials and methods
The plant material was recollected from the wild plants in vicinity of the Cruz Verde plateau in July, 2014. The collection site was located at an altitude between 3,300 and 3,400 m a.s.l. on highly steeped terrain and corresponding to the East Andes of Colombia in the municipality of Choachí, Cundinamarca province.
In 17 mother plants of each T. floribunda (3,216 m a.s.l., 4°33'53.0" N and 73°58'36.9" W) and C. bracteata (3,246 m a.s.l., 4°33'51.22" N and 73°58'58.5" W), primary branches measuring from 20 to 40 cm in adult vegetative state were selected (without the presence of fruits), with no incidence of apparent diseases or damage due to the presence of insects.
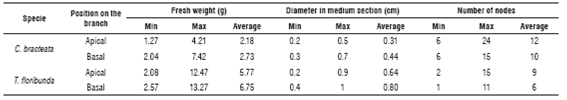
Prior to cutting preparation, the branches were washed in soapy water and then stirred with abundant running water. All cuttings had an average length of 12 cm. Two-thirds of the expanded leaves in each cutting were cut off with disinfected scissors in order to avoid cutting dehydration. Fifty two cuttings of each species were taken to measure the initial characteristics (Tab. 1).
In both species, the apical cuttings had shorter internodes and, therefore, a higher number of nodes than the basal cuttings, where the internodes were longer. The branches of C. bracteata presented a narrowing in the apical part, which contributed to a lower weight of this segment of the branch (Tab. 1).
To the base of cuttings, naphthalene acetic acid (NAA) (0.4% Hormonagro 1®; Colinagro, Bogota, Colombia) was applied. Kaolin was used to prepare a mixture with NAA in order to produce the concentration of 0, 200, 500, or 1,000 mg L-1 NAA; the mixture was applied as a paste to the cutting base.
Promix PGX® peat-based substrate in a 1:1 (volume/volume) mixture with burnt rice husk was used as the growth medium. Previously to planting the growth medium (pH 5.96) was disinfected with a broad-spectrum fungicide with carboxin active ingredient (200 g a.i./kg) in a dose of 1 g L-1 and then washed abundantly with water.
The study was developed in the greenhouses of the Faculty of Agricultural Sciences (Universidad Nacional de Colombia, Bogota, Colombia). The cuttings were placed in plastic trays of 8x8x8 cm cells under black plastic mesh allowing 30% light transmission to the cuttings. Light conditions of 125.83 µmol m-2 s-1 (quantum sensor Li-189; LICOR Inc., Lincoln, NE, USA), 17.3°C average temperature (max. 32.4°C, min. 10.3°C) and 72.4% average relative air humidity (max. 96.6%, min. 34.6%) (DataLogger WatchDog® 100 T/RH, Spectrum Technologies, Aurora, IL, USA) were monitored. During the experiment, the cuttings were watered twice a day by microspray up to field water capacity.
The variables evaluated before the establishment phase were the number of nodes, cutting diameter, and cutting fresh weight. After 120 d of the experiment, the variables determined were dry and fresh weight of cuttings, root fresh and weight, shoot fresh and dry weight, and number of shoots and roots. This last measurement was done through ImageJ (http://imagej.net/ImageJ). The dry weight of roots and shoots was determined by drying the plant organs at 65°C for 48 h. As indirect variables percentages of cutting survival (survived cuttings/planted cuttings * 100%), rooting (rooted cuttings/planted cuttings * 100%) and sprouting or emission of new shoots (sprouted cuttings/ viable cuttings * 100%) were determined at the end of the study. A histological analysis was carried out based on the methodology of permanent assembly of roots in paraffin of Sass (1951) using basal segments of cuttings, in order to understand the origin of adventitious root formation and justify the success of rooting in cuttings.
A randomized design with two factors was implemented: cutting position on the branch and concentration of NAA applied to the cutting. The first factor had two levels (apical and basal position) and the second factor had four levels (0, 200, 500, and 1,000 mg L-1 of NAA). The eight treatments corresponding to four NAA concentrations and two cutting positions consisted in 13 replicates each for both species; each replicate was represented by one cutting.
Each species was evaluated separately following the same parameters. The assumptions of normality and homogeneity of variance were used; in case they were not met, Box and Cox parameters were used for data transformation. An Anova test was performed for the analysis of treatments using SAS 9.1.3 (SAS Institute, Cary, NC). If significant differences were found, Tukey's test (P≤0.05) was applied to identify the treatments that differed from each other.
Results and Discussion
Formation of new shoots
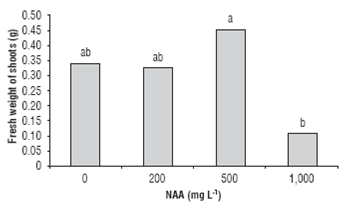
At 120 d of the experiment, the cuttings of T. floribunda did not produce new shoots. According to the analysis of variance, in cuttings of C. bracteata, it was observed the effect of NAA concentration on sprouting percentage (Fig. 2) as well as on fresh weight of shoots (Tab. 2).
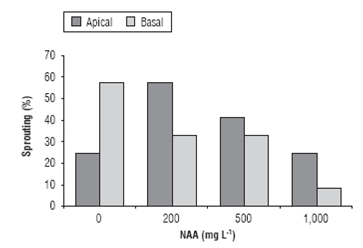
FIGURE 2 Percentage of sprouting in Cavendishia bracteata cuttings treated with different concentrations of NAA.
TABLE 2 Effect of NAA application and cutting position on the weight of shoots, roots and total weight of cuttings of T. floribunda and C. bracteata at 120 d.

Significance levels ***P<0.001, **P<0.01,*P≤0.05; NS: Non-significant differences.
From the transformed values of the variable number of shoots, according to analysis of variance, T. floribunda did not produce new shoots in any treatment unlike C. bracteata. This can be explained by the differences in shoot production capacity, which varies according to the particular genotype of each parent plant (Aparicio et al., 2009). Also, application of auxins in excessive concentrations can inhibit the development of new shoots due to the stimulation of ethylene synthesis (Hartmann et al., 2010), although no production of new shoots was equally detected in control T. floribunda cuttings with no NAA applied.
According to the analysis of variance, the effects of cut-ting position, NAA concentrations, and their interaction on shoot formation in cuttings were evidenced. The basal cuttings of C. bracteata sprouted better than the apical ones in the control treatment (0 mg L-1 NAA), presenting a sprouting percentage of 58.3% (Fig. 2). This may be due to the elimination of apical dominance, a natural process, where the apex inhibits growth of the buds located in the middle and basal areas of the branches, thus, avoiding excessive growth of axillary branches due to the relationship among auxins and strigolactones/cytokinins (Shimizu-Sato et al., 2009).
C. bracteata presented a sprouting in the apical cuttings under the application of NAA, with 58.3, 41.7, or 25% for the concentrations of 200, 500, or 1000 mg L-1 NAA, respectively. Otherwise, when applying NAA to the basal cuttings, these had a lower percentage of sprouting compared to the control. The results demonstrated that the effect of NAA on cutting ability to form new leaf area depends on the position of cuttings on mother plants (Tab. 2 and Fig. 2).
In cuttings obtained from the base of the branch, the control treatment had the highest sprouting of 58.3%; it was also observed that increasing concentrations of NAA resulted in reduced sprouting. In general, at 1,000 mg L-1 NAA, the percentage of shoot formation decreased independently of the position of cutting on the mother plant (Fig. 2), which indicates that high concentrations of NAA had an inhibitory effect on sprouting. According to Hart-mann et al. (2010), the application of auxins on cuttings at high concentrations may inhibit the development of leaf buds. As for the fresh weight of shoots, according to the analysis of variance, it presented significant differences with the use of 500 mg L-1 NAA (Fig. 3), suggesting that when treated with 500 mg L-1 NAA, in C. bracteata, there was a higher accumulation of water and photoassimilates in leaves.
Survival and rooting of cuttings
The cuttings of C. bracteata had a higher percentage of survival than T. floribunda (Fig. 4). This can be attributed to the fact that T. floribunda has larger diameter of cuttings, especially the basal ones (Tab. 1), which were more susceptible to dehydration than those of C. bracteata and, therefore, had the higher mortality rates in all treatments. The cuttings of C. bracteata had the higher percentages of survival (75% basal and 83.33% apical) than those of T. floribunda (33.3% basal and 58.3% apical) (Fig. 4). No differences were found to establish an optimal range of NAA concentrations that would facilitate the survival of cuttings for both species.

FIGURE 4 Survival percentage of apical and basal cuttings of C. bracteata (A) and T. floribunda (B) at different concentrations of NAA.
In general, rooting of cuttings was more successful in C. bracteata than in T. floribunda (Fig. 5). For the latter species, the basal cuttings treated with 500 or 1,000 mg L-1 NAA, obtained the same percentage of rooting (16.7%), however, better results were obtained when NAA was not used. This occurred similarly in the apical cuttings of T. floribunda since no positive effect of NAA treatments was observed, which indicates that NAA applied in concentrations ranging from 200 to 1,000 L-1 was not necessary for rooting in T. floribunda. As for C. bracteata, the apical cuttings had a higher percentage of rooting with values between 58 and 75%, compared to the basal cuttings with values between 50 and 58%. The cuttings selected from the base of the branches were closer to the main stem, a position that might provide a higher accumulation of carbohydrates at the base of the shadow-grown branches and, thus, be of further importance for the formation of adventitious roots (Murray et al., 2013). In both species, the apical cuttings had a greater number of nodes implying a higher synthesis of auxins compared to the basal ones. The position of cutting on the mother plant influenced the percentage of rooting (Fig. 5).

FIGURE 5 Rooting percentage of apical and basal cuttings of C. bracteata (A) and T. floribunda (B) treated with different concentrations of NAA.
The diameter and number of nodes may influence on the rooting ability of cuttings. According to Hartmann et al. (2010), rooting may be influenced by the type of branch from which the cuttings are taken due to its chemical composition. This was observed in Rhododendron sp. (Ericaceae), where cuttings obtained from thin branches showed a better rooting (Hartmann et al., 2010). Otherwise, the cuttings obtained from the basal branches might have greater capacity to root than the ones obtained from the terminal branches (Baldini, 1992). This is due to the accumulation of carbohydrates in the base of the branches in woody stems older than one year, allowing formation of root primordia due to promoter substances originated from buds and leaves (Hartmann et al., 2010).
Number and weight of adventitious roots
In C. bracteata, with NAA 200 mg L-1 it was obtained a higher number of roots compared to the other treatments (Fig. 6). In T. floribunda, apical cuttings treated with 1,000 mg L-1 NAA obtained a higher number of roots (Fig. 6), which shows that the effect of auxin concentration and its ability to produce roots depended on the species. For T. floribunda, at the lowest NAA dose corresponding to 200 mg L-1, total mortality of both apical and basal cuttings was observed.

FIGURE 6 Number of adventitious roots in C. bracteata (A) and T. floribunda (B) cuttings treated with different concentrations of NAA. The lower base of the box plot corresponds to Q1, the upper base corresponds to Q3, the line inside the box is the median or Q2 and the point is the arithmetic mean, the upper limit corresponds to Q3 + interquartile range (Q3-Q1) * 1.5, the lower limit corresponds to Q1-interquartile range (Q3-Q1) * 1.5.
Similarly, in T. floribunda, an increase in fresh and dry root weight with increasing NAA concentration was observed, since the treatment of 1,000 mg L-1 yielded a significantly higher root weight, presenting very significant differences with values of 0.65 g and 0.18 g, respectively (Tab. 2 and Fig. 7), as well as the increased number of roots. According to the analysis of variance, this variable was very significant for apical cuttings with an average of 0.35 g for fresh weight and 0.09 for dry weight (Tab. 2 and Fig. 7). Durán-Casas et al. (2013) reported that at concentrations of 500 and 1,000 mg L-1 IBA in native Andean species Macleania rupestris there were obtained up to two to three times as many roots as in control, similarly, these treatments had the highest dry root weight.

FIGURE 7 Fresh (A) and dry (B) root weight of C. bracteata cuttings; fresh (C) and dry (D) root weight of T. floribunda cuttings. Treatments with the same letter are not significantly different according to the Tukey's test (P≤0.05).
Both species belong to the tribe Vaccinieae and naturally grew in the same environments, but their cuttings have different rooting capacity; this aspect was earlier shown for various woody species. Thus, multiplication of Disterigma alaternoides by cuttings had the higher potential than that of Vaccinium floribundum, since it was almost twice easier to root under the same conditions (Magnitskiy et al., 2011). In some species, high concentrations of auxins are necessary at the stages of rooting induction and further growth of root primordia (Hartmann et al., 2010). For example, Biasi et al. (1990), in semi-hardwood cuttings, with a concentration of 2,000 mg L-1 IBA, obtained a higher number of roots. However, C. bracteata cuttings treated with 200 mg L-1 NAA had the highest (0.13 g) fresh weight of roots (Fig. 7). This coincides with results found for V. floribundum, where applications of NAA between 200 and 400 mg L-1 were favorable for the rooting process (Magnitskiy et al., 2011).
The effect of auxins on increasing the number of roots and accelerating formation of roots has been observed in different plant species. Burgos et al. (2005) observed a favorable action of hormone application on the total aerial biomass, root biomass, fibrous root biomass, and root length in Manihot esculenta.Olival and López (2005) found positive effect of 100 mg L-1 NAA application on diameter, number of roots, and increased rooting percentages (24.47%). However, when NAA concentration increased to 300 mg L-1, the rooting percentage decreased (Olival and López, 2005). Contrary results were observed in plants of the genus Bougainvillea (Nyctaginaceae), an exuberant subtropical vine, where the basal ends of the cuttings were immersed in NAA solutions of 2,000, 4,000, or 6,000 mg L-1 (Noor-un-nisa, 2013). The best result was obtained from the treatment at 6,000 mg L-1 NAA, with the highest number of shoots, shoot length, root length and number (Noor-un-nisa, 2013). This data confirm that the response to the hormone application is determined by the species studied. Additionally, the cuttings of woody species thicker than 1 cm in diameter might have been rooted better, because at larger diameter there are higher amounts of reserves and, therefore, a higher probability of rooting.
Total fresh and dry weight of cuttings
For C. bracteata and T. floribunda, there was an effect of NAA concentration on both total fresh weight and total dry weight of cuttings at the end of the experiment (Fig. 8). For these two variables in C. bracteata, there were significant differences of the treatment of 500 L-1 NAA compared to the treatment of 1,000 L-1, however this last dose resulted in the highest fresh and dry weights of T. floribunda cut-tings being significantly higher in comparison with doses of 0 and 500 L-1 (for fresh weight) and dose of 0 L-1 (for dry weight).
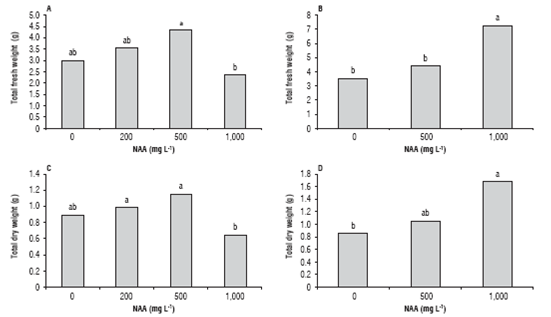
FIGURE 8 Total fresh weight of cuttings of C. bracteata (A) and T. floribunda (B) at 120 d. Total dry weight of cuttings of C. bracteata (C) and T. floribunda (D) at 120 d. Treatments with the same letter are not significantly different according to the Tukey's test (P≤0.05).
The fresh weights in C. bracteata increased for both apical (2.71 g) and basal cuttings (3.94 g), since their initial weights were 2.18 g and 2.73 g, respectively, showing a significant effect (P<0.01) of cutting position on this variable (Tab. 2 and Fig. 8). For T. floribunda cuttings, there were no significant effects of cutting position on the final fresh weight of cuttings (Tab. 2); however, there was a decrease in final fresh weights of cuttings after 4 months (4.38 g apical and 5.8 g basal) compared with the initial values (5.77 g apical and 6.75 g basal) (Fig. 8). This data suggests that T. floribunda cuttings were susceptible to dehydration independently of their position on the mother plant; the present feature, apparently, contributed to the low percentage of cutting survival in T. floribunda.
Histological analysis of the origin of adventitious roots
The transverse histological sections made at the base of cuttings of C. bracteata and T. floribunda show that the adventitious roots originated from parenchyma cells of the cuttings (Figs. 9 and 10). For both T. floribunda and C. bracteata, it was observed that the newly formed adventitious roots had an endogenous origin according to Rudall (2007). This type of root formation occurs from the mitotic divisions of cambium cells and vascular tissues that differentiate into isodiametric cells that give rise to the root primordia (da Costa et al., 2013).
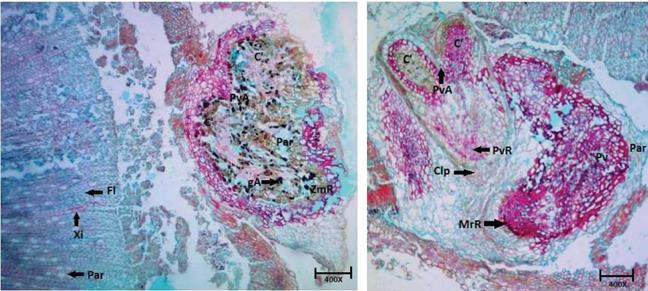
FIGURE 9 Transverse histological sections of the stem base of C. bracteata. Fl (Phloem). Xi (Xylem). Par (Parenchyma). ZmR (Root meristematic zone). gA (Starch granules present in the parenchyma). PvA (Apical vegetative point). C' (Leafy primordia). Pv (Vegetative point). PvA (Apical vegetative point). PvR (Root vegetative point-adventitious root primordia). Clp (Caliptra). MrR (root meristem)

FIGURE 10 Transverse histological sections of the stem base of T. floribunda cuttings. Esc (Sclerenchyma). Par (Parenchyma). Ctz (Cortex). PvR (Root vegetative point). Xi (Xylem). Fl (Phloem). MrR (Root meristem-adventitious root emission).
In stem cuttings, root primordia usually originate from the epidermis, cortical parenchyma, radial parenchyma, vascular cambium, and phloem parenchyma (Hartmann et al., 2010). The species with easy rooting, as appeared to be the case of C. bracteata, develop their adventitious roots from the cells of parenchyma. The cells of meristematic zone are organized to form root primordia in the cortex and then a vascular connection is created between the root primordia and the vascular system of a cutting (Hartmann et al., 2010). In C. bracteata cuttings, the vegetative point that gives origin to the root meristem (MrR) was located near parenchyma (Fig. 9). Additionally, it was possible to identify the vegetative radicular (PvR) point covered by the caliptra and the apical vegetative point (PvA) that gives rise to formation of two leafy primordia (Fig. 9).
The formation of adventitious roots originated in woody species from latent root primordia in the stem nodes is stimulated by the high auxin concentrations in the adjacent meristematic cells of the stems (Hartmann et al., 2010). Separated cutting from the branch allows endogenous aux-ins and carbohydrates accumulation in its base, increasing respiration; contributing to cell division and development of root primordial. In addition, the wound at the cutting base stimulates ethylene synthesis that contributes to transport, signaling, and accumulation of auxins and, thus, participates in root formation (Da Costa et al., 2013; Lewis et al., 2011).
Similarly, for T. floribunda, emission of adventitious root primordia occurred from the cells of parenchyma (Fig. 10). In general, in T. floribunda, there was less adventitious root production than in C. bracteata (Fig. 6). In addition, in T. floribunda, it was evidenced the presence of angular sclerenchyma, a tissue characterized by thickened walls and thick cytoplasm (Fig. 10). According to Hartmann et al. (2010), the production of adventitious roots depends on the physiological and genetic conditions of species. Juvenile cuttings, in woody plants, often present better rooting than adult cuttings (Murray et al., 2013), such as the ones used in the present study. Particularly, root formation may be reduced due to a ring of highly lignified sclerenchyma in the stem that impedes the rooting process or presents constraints for the newly formed roots to emerge. This tissue is usually present in mature stems (Fahn, 1985) and can obstruct the emergence of roots that originated in the cambium and phloem (Lodama et al., 2016).
Induction of root primordia in cuttings occurs once the auxins are applied exogenously and, in general, this process begins during the first 24 h after the application (Hartmann et al., 2010). In this period of time, cellular dedifferentiation occurs, a process observed before the induction of root primordia in competent cells, such as in apple tree (De Klerk et al., 1999). Further research should be dedicated to elucidate the biochemistry of root formation, including the competence of cells to initiate root primordia, in Ericaceae species that can't reach rooting processes easily, such as T. floribunda.
Conclusions
Although both species grew in similar environments, the application of auxins to the cuttings had different effects in these plants, both in shoot and root production and percentage of cutting survival at 120 d. In C. bracteata, the cutting position on the branch affected its sprouting and rooting, with apical cuttings presenting the best performance with auxin application. With 1,000 mg L-1 NAA the sprouting in C. bracteata cuttings decreased regard-less of cutting position on the mother plant. The rooting of cuttings was more successful in C. bracteata than in T. floribunda. NAA applications between 200 to 500 mg L-1 were more suitable for the propagation of C. bracteata due to the higher percentages of rooting and shoot production. For propagation of T. floribunda, the use of apical cuttings without the application of auxins is recommended. The adventitious roots in cuttings of both species originated from parenchyma cells of the stem.




![Organogénesis in-vitro using three tissues types of tree tomato [ Solanum betaceum (Cav.)]](/img/pt/prev.gif)