Introduction
Passiflora L. is the most important genus of Passifloraceae with around 576 species, mainly distributed throughout the Americas and to a lesser extent in tropical and subtropical Australia, New Zealand and Southeastern Asia (Ocampo et al., 2010). Many species are cultivated for their edible fruits, as ornamentals, or for their medicinal properties (Yockteng et al., 2011). The yellow passion fruit (P. edulis f. flavicarpa Degener) is by far the best known and economically most important species of the genus, with a world production estimated at approximately 805,000 t (Passionfruitjuice, 2015). Maracuja is the name adopted in its country of origin Brazil, and known around the world under the names of yellow passion fruit, maracuyá, parchita, chinóla and calala. The yellow passion fruit is a self-incompatible allogamous plant pollinated by large wasps (Arias et al., 2014), with 2n=18 chromosomes (Snow and MacDougal, 1992), and a size genome of 1.26 pg (Yotoko et al., 2014).
As the commercial cultivation of P. edulis f. flavicarpa started at its place of origin in tropical America with materials repatriated from Hawaii, propagation by seedlings became the most employed method. In Colombia, farmers have contributed to the domestication and the development of the yellow passion fruit, as they have established new cultivation practices according to their knowledge and experience, including manual pollination, fertilization and pruning practices. These cultural practices together with the increase in production areas and the abundance of natural pollinators magnified the genetic variation, widening the basis for later breeding programs. This has already been taking place for many years in Brazil that has become, according to IBGE (2014), the first yellow passion fruit production and consumption country worldwide with up to 780,000 metric tons.
In Colombia, yellow passion fruit or "maracuyá" was introduced in 1960's to Valle del Cauca department with seeds from Hawaii (USA), and currently reports 5,500 ha of cultivated areas located from 300 to 1,450 m a.s.l., with a production of 17-20 t ha-1 (Agronet, 2016). In Colombia the departments of Valle del Cauca and Huila concentrate more than 50% of cultivated area with a production of approximately 60.000 t of yellow passion fruit. Fresh yellow passion fruit is an important commodity for domestic consumption in diverse preparations as juices, sherbets or ice cream, and a 65% of the Colombian production is processed as frozen juice for the international market. A major limiting factor in the crop's development in Colombia is the large number of pests and diseases, with considerable negative effects on production (Ocampo et al., 2013). Moreover, in the country's fruit nurseries, plant health parameters regarding production of planting material are not controlled or strict. An additional problem lies in the lack of breeding programs that offer cultivars of higher genetic quality that may respond to adverse problems that affect the yellow passion fruit crops. Some plant responses that are desired for commercial production in yellow passion fruit are: early flowering, improved yield, pests resistance (Neohydotothrips signifier Priesner and Dasiops inedulis Steyskal), diseases resistance (Fusarium oxysporum and Soybean Mosaic Virus - SMV) and drought tolerance. In spite of the lack of basic knowledge on the yellow passion fruit's genetic resources, complex technical approaches have been explored directly in yellow passion fruit, including interspecific hybridizations (Payán and Martín 1975; Ocampo et al., 2016) and genetic transformation (Manders et al., 1994; Monteiro et al., 2011) to generate information that could be used to guide breeding programs.
The genetic variability in the genus Passiflora is very wide both within the genus and also within the most cultivated species (Ocampo and Coppens d'Eeckenbrugge, 2017). In commercialized species such as yellow passion fruit, growers carry out phenotypic or mass selection practices when establishing or renewing new plots in function of their observations, or of a phenotype imposed by local or international markets (Ocampo et al., 2013). Seeds are collected from a small number of good quality fruits plucked from one or two high performing plants. Given the size of the plots, the total population is small and the selection intensity is low, especially if the plantation's renewal cycle is rather long. This practice as well as seed exchanges among farmers maintain considerable variability in the populations. The first work of breeding program took place in the developed tropical and subtropical regions where the commercial cultivation of P. edulis was initiated, but with very limited genetic resources and without even knowing the existing variation. These few institutional efforts were concentrated on the clonal propagation of hybrids between yellow and purple passion fruits obtained on a very narrow genetic base in Australia, Hawaii and Florida (Knight, 1972; Winks et al., 1988). The most advanced Brazilian breeding programs in yellow passion fruit have used progeny-testing to get synthetic populations, aiming at developing crop material with better productivity, quality and homogeneity, while maintaining sufficient genetic diversity for efficient cross-pollination (Oliveira et al., 2008; Reis et al., 2011, 2012). Furthermore, several cultivars have been proposed from year 2000 to the present day for consumption in natura and for the agroindustry in Brazil (Meletti et al., 2000, 2005; Nascimento et al., 2003; Cerqueira et al., 2014a), with a great impact on fruit production and quality improvement in the recent years (Cerqueira et al., 2016).
Molecular and phenotypic markers have become powerful tools to understand the structure and evolution of species diversity, as well as in plant breeding and in conservation of genetic resources related activities (Faleiro et al., 2005; Reis et al., 2012). The first study of genetic variation in the genus Passiflora using DNA markers was based on RAPD, cpDNA and RFLP reported by Fajardo et al. (1998) and Sánchez et al. (1999), to verify relationships between species of the subgenera Passiflora, Tacsonia, Decaloba and Distephana. Other studies with RAPD markers (Aukar et al., 2002; Cro-chemore et al., 2003) showed a higher uniformity among accessions of P. edulis f. flavicarpa from Brazil. Segura et al. (2002) and Ocampo et al. (2004) investigated the genetic relationships among cultivated Passiflora species of the subgenera Passiflora and Tacsonia using AFLP markers. The results showed high diversity at the intraspecific level and among closely related species as P. edulis f. flavicarpa.
Microsatellite or SSRs markers are short stretches of repeated DNA of di- or tri-nucleotide repeats spread throughout the genomes that are useful tools to study genetic diversity in multiple crops (Ocampo et al., 2007; Blair et al., 2012; Hasnaoui et al., 2012). These markers provide many advantages over many other DNA markers as they are generally abundant, highly polymorphic, codominant, and with up to 25 alleles that are common at an individual locus (Billote et al., 1999). The first isolation and characterization of microsatellite markers in Passiflora were reported by Oliveira et al. (2005) and Padua et al. (2005) from yellow passion fruit (P. edulis f.flavicarpa) and fragrant granadilla (P. alata Curtis) from Brazilian accessions. New microsatellite markers for wild and commercial species of Passiflora reported by Cerqueira et al. (2012, 2014a) confirmed that these are useful tools to understand the mating system and hybridization within the genus. A study performed by Santos et al. (2011) using ISSR markers in P. edulis (purple and yellow type) and P. alata accessions showed that there is no structure in the populations evaluated, although the results provide practical information for parental selection to assist breeding. A more comprehensive analysis of the genetic diversity recurrent selection assessments of yellow passion fruit based on molecular and agronomic data carried out by Reis et al. (2011, 2012), indicated the possibility of applying the combined data to optimize genetic gain for the traits under selection. In Colombia, Ortiz et al. (2011) did not observe polymorphism using 17 SSR primers in commercial plantations of purple passion fruit (P. edulis f. edulis Sims), suggesting that the cultivated germplasm came from the same origin. Other investigations carried out in Colombian P. ligularis (sweet granadilla) accessions, suggest that the germplasm cultivated in the country shows a high variability (He=0.96) with a slight genetic structure (Bernal et al., 2014). In contrast, genetic variation of commercial passion fruit accessions from Brazil showed that P. edulis f. flavicarpa (He=0.50), P. cincinnata (He=0.52) and P. setacea (He=0.36) show moderate to low variability (Cerqueira et al., 2014b) using inter-simple sequence repeat (ISSR) markers. These DNA markers have also contributed to the identification of groups of preferential accessions of yellow passion fruit (P. edulis f. flavicarpa) with genetic resistance to diseases (Cerqueira et al., 2015). Recently, Silva et al. (2016) used microsatellite markers to characterize a progeny derived from the third cycle of recurrent selection of yellow passion fruits, allowing the separation of individuals into three groups and generating relevant information for further breeding programs.
Colombia is the third producer worldwide of yellow passion fruit after Brazil and Ecuador. Despite this potential, there are few research efforts carried out related to the knowledge of genetic variability that could be a starting point for future breeding programs. Additionally, the National Germplasm Bank of Colombia, managed by the Corporacion Colombiana de Investigacion Agropecuaria (Corpoica) does not report accessions preserved of P. edulis f. flavicarpa. However, a first study performed by Ocampo et al. (2013) based on the exploration of the genetic variability of yellow passion fruit, establishes the basis for a breeding program from 44 accessions collected in commercial plantations and characterized with emphasis on fruit quality. In this context, it is necessary to continue with other genetic studies to extract further information about the current genetic variability and the relationships between individuals from the germplasm gathered. For these reasons, the main goal of this study was to assess the genetic variability and to decipher the population structure of 51 commercial yellow passion fruit accessions, in order to provide the necessary information for an efficient management and use of these genetic resources for prospective conservation, selection and breeding programs.
Material and methods
Plant material and study area
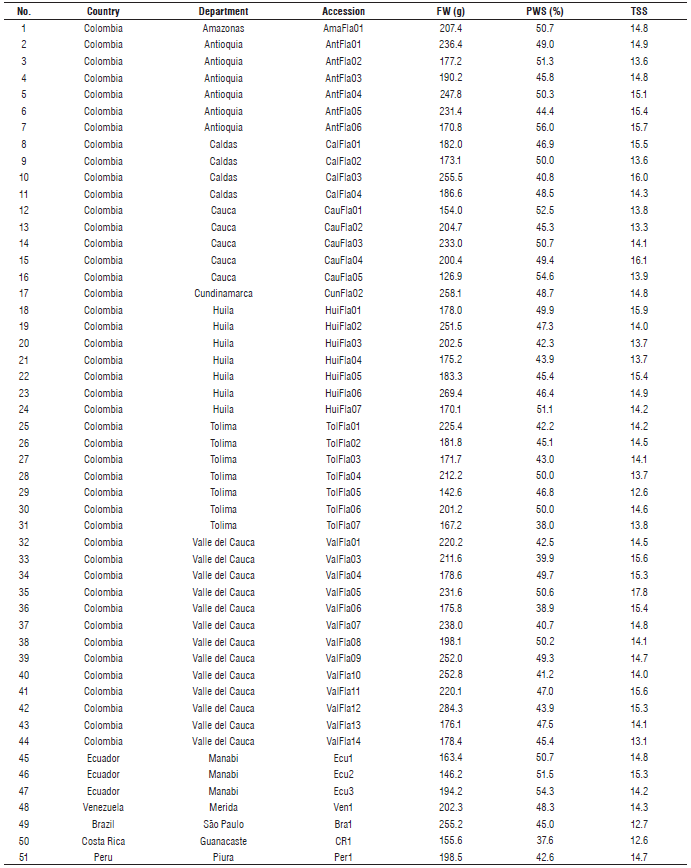
During the research study conducted by Ocampo et al. (2013) the germplasm used in this study was collected. The germplasm set included 51 yellow passion fruit accessions, 44 of which were obtained from commercial plantations located in different geographical regions in Colombia, and seven accessions from other countries (Tab. 1). The accessions were planted in the Casa Luker farm (5°04'25.95" N; 75°41'4.71" W) located in the department of Caldas (Colombia) at 1,023 m a.s.l., with an average annual temperature of 23°C and an average annual precipitation of 2,200 mm. Each accession is composed by two individuals, which come by open pollination and are considered as a half-sib within each accession.
DNA extraction and microsatellite analysis
DNA was extracted from fresh leaves of adult plants cultivated under field conditions, using the methodology of Doyle and Doyle (1991). Extractions were assessed by electrophoresis in agarose gel at 1% in 0.5X TBE buffer stained with etidium bromide (2 μg mL-1). The results were recorded using a photo-documenting UVP GelDoc-ItTMSystem (UVP, Upland, USA) and analyzed with LS Works® Vision Image Acquisition and Analysis Software (Imagingsystem, USA). DNA was quantified using the Nanodrop 1000 Spectrophotometer equipment and the ND-1000 V3.7.1 program (ThermoScientific, USA).
The study was carried out with 10 polymorphic microsatellite markers previously selected (Oliveira et al., 2005). PCR reactions were performed in a TC9600-G MultiGene Gradient Thermal Cycler (Edison, NJ, USA) with a final volume of 15 μL in a mixture containing 1X buffer PCR, 2 mM of MgCl2, 0.2 mM of dNTP, 0.2 μM of each primer, 1U of Taq DNA polymerase and 10 ng of genomic DNA. Ther-mocycling program consisted of an initial denaturation at 93°C for 5 min, followed by 36 cycles of 40 s at 94°C, 40 s at 52-56°C (depending of each primer annealing), 50 s at 72°C and with a final extension of 10 min at 72°C. The amplification products were first visualized on ethidium bromide - stained agarose gels (2.5%) in 0.5X TBE for assessing the amplified product. Then, these were run in electrophoresis on 8% denaturing polyacrylamide gels in 1X TBE at 200 W for 90 min and stained with silver nitrate. The bands reveled identified with a white light lamp (Hoefer Macro Vue Vis_45) and photographed with a Samsung® S760 (7.2 Mp) digital camera. The sizes were established using a 10-bp DNA Hyperladder V (BIOLINE, California, USA) and were then quantified with the software Vision Works LS Image Acquisition (UVP, Upland, USA).
Statistical and genetic analyses
The gel images were coded under allelic sizes of the diverse observed bands. The allelic richness, frequency, observed (Ho) and expected heterozygosity (He), polymorphism information content (PIC), fixation index (F) and the Hardy-Weinberg test (HWE) for each locus were calculated and executed with the Genetix 4.05 program (Belkhir et al., 1996-2004). The relationships between accessions and genetic distance were estimated with Nei's coefficient (Nei, 1978) and to these results a neighbor joining cluster analysis (Saitou and Nei, 1987). Bootstrap analysis with 1000 replicates (Felsenstein, 1985) was performed to get the confidence level of the tree. Additionally, all alleles per locus were georeferenced for spatial distribution following van Zonneveld et al. (2012). A grid for allelic richness parameter was generated using the DIVA-GIS 7.5 software with a cell size grid of 0.05 minutes (which corresponds to approximately 0.1 km in the study area) and applying a circular neighborhood with a 0.5 degrees diameter (corresponding to approximately 55.5 km).
To assess the genetic structure of the accessions a Bayesian clustering analysis was also applied to estimate the potential number of subpopulations (K) that may exist in the overall individual samples. The used program Structure was v.2.3.4 (Pritchard et al., 2000). The number of subpopulations (K-value) was set from 2 to 10, using 20 independent runs with a burn-in period of 100,000 steps, and afterwards 200,000 Monte Carlo Markov Chain (MCMC) interactions after burn-ins, following the admixture ancestry model and correlated allele frequencies, which is appropriate for self-incompatible allogamous species (Porras-Hurtado et al., 2013). Results of runs with the highest ln Pr (G|K) value of the 20 runs were chosen and presented as bar plots according to the Evanno et al. (2005) method.
Results
Microsatellite analysis SSR
A set of six out of 10 microsatellite markers previously isolated and characterized by Oliveira et al. (2005) in yellow passion fruit detected polymorphism in the 51 accessions evaluated, with a total of 58 alleles revealed (Tab. 2). The number of alleles per locus detected ranged from 6 to 18 with an average of 9.66 (Tab. 2). The richest allelic markers were AY768785 and AY768786 with 18 and 11 alleles respectively, and the average size of each allele was 227 bp showing a range of 96-356 bp. Among the alleles detected, nine were identified as private alleles and 31 as rare (frequency ≤10%) distributed particularly in accessions from Huila (HuiFla 01, 06 and 07), Valle del Cauca (ValFla 07, 08, 09, 10, 13 and 14), Tolima (TolFla 01, 02 and 04), Antioquia (AntFla 01, 04 and 06), Caldas (CalFla 01), Amazonas (AmaFla 01), Cundinamarca (CunFla 02), Cauca (CauFla 01), Ecuador (Ecu), Brazil (Bra), Costa Rica (CR) and Peru (Per). On the other hand, figure 1 presents the spatial distribution of the allelic richness parameter for all loci found in the Colombian accessions, showing clearly that a higher number of alleles were found in the majority of Valle del Cauca accessions (8 to 14), while the allelic richness parameter in other departments as Huila, Antioquia and Tolima showed a maximum of 7 alleles. The polymorphic information content (PIC) varied between 0.63 (AY768790) and 0.92 (AY768785) with an average value of 0.74 for six of the selected markers (Tab. 2). In accordance with Botstein et al. (1980) these are highly informative (≥ 0.50) for codominant molecular markers.
TABLE 2 Microsatellite markers used indicating their primer sequence, annealing temperature (Ta), number of alleles (Na), allelic composition, allele size range, polymorphism information content (PIC), heterozygosity (H0, H e ), Chi-Square Tests for Hardy-Weinberg Equilibrium (P≤0.05) detected in the 51 yellow passion fruit accessions.
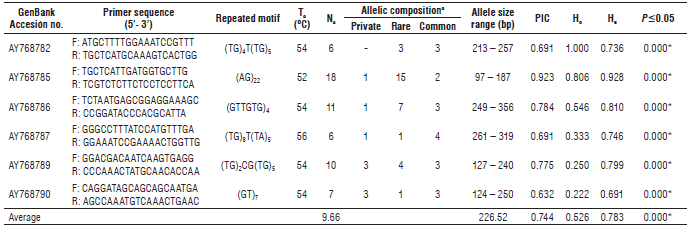
a private allele frequency <1%; rare allele ≤10%; and common allele ≥10%.
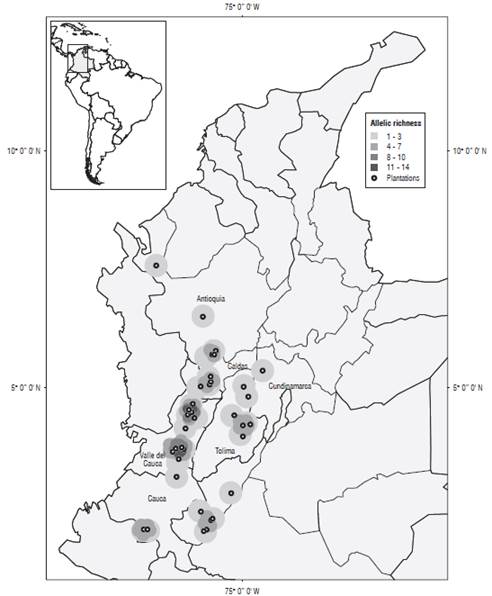
FIGURE 1 Spatial distribution of yellow passion fruit plantations modeled based on allelic richness. Red areas indicate high concentration of allelic richness (11 to 14). The accession from the Amazon region (1 to 3 alleles) is not shown on the dot map.
The average of observed (Ho) and expected (He) hetero-zygosities was 0.52 (0.22 to 1.00) and 0.78 (0.69 to 0.92), respectively (Tab. 2). An exact test for the Hardy-Weinberg equilibrium (HWE) law showed significant deviations for all loci evaluated. The AY768785 and AY768782 loci revealed the highest information because 80.6 to 100% of the individuals were heterozygous (Ho) for the same alleles, and demonstrated an excess of heterozygous plants. In contrast, less than 25% of the individuals were heterozygous at the AY768789 and AY768790 loci (Ho=0.22 to 0.25, respectively), indicating an inbreeding tendency.
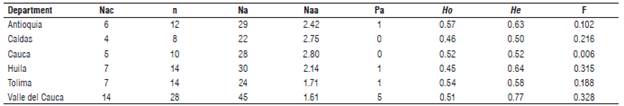
Among the higher yellow passion fruit producer departments in Colombia, Valle del Cauca and Huila showed the highest number of alleles, i.e. 45 and 30, respectively (Tab. 3). In contrast, the average number of alleles (Naa) on six loci evaluated showed that the accessions of the departments of Caldas and Cauca had the best averages (2.75 and 2.80), whereas Valle del Cauca accessions only showed an average of 1.61 alleles. However, within latest accessions 56% of private alleles were identified (Pa, 5). The diversity parameters among accessions of the mainly producer departments of yellow passion fruit in Colombia showed a range of expected heterozygosity (He) from 0.50 (Caldas) to 0.77 (Valle del Cauca). A deficit of heterozygosity (Ho < He) was observed for the most of the departments, whereas Cauca accessions shows Hardy-Weinberg Equilibrium (Ho=He). The fixation index (F) average observed between the different departments was 0.193 and indicating that Valle del Cauca (0.328) and Huila (0.315) show the greatest differentiation between its accessions.
Genetic diversity
The average total genetic distance observed between and within accessions of the same geographic origin was 0.68, with values ranging from 0.19 to 0.98 (Tab. 4). The smallest distances were found between individuals of the same origin as in the case of Costa Rica (0.19) and Amazonas (0.27). In contrast, the average distance between accessions of different geographic origins reached 0.74, the most distant ones related to otherswith the Amazonas (0.81), Venezuela (0.78) and Valle de Cauca (0.78) accessions.
TABLE 4 Genetic distances according to Nei (1978) among the 51 yellow passion fruit accessions evaluated per geographic region. Bold values indicate distances >0.75.

The genetic relationships between the yellow passion fruit accessions studied are shown in a cluster analysis using the neighbor joining method based on the SSR data (Fig. 2). The dendrogram showed three main groups poorly supported (bootstrap <50%), with a slight geographical structure and a high differentiation between individuals of the same accession. The accessions from Huila (HuiFla), Antioquia (AntFla) and Valle del Cauca (ValFla) are represented in the three groups, and some associations between individuals of the same origin can be identified, as ValFla01, 02, 06, 08, HuiFla04, 05, and AntFla03, 05. In group I, the accessions from Ecuador (Ecu), Brazil (Bra), Costa Rica (CR) and Peru (Per) are mixed with the accessions from Cauca (CauFla). In the nether branch of the third group (III) the Tolima (TolFla) accessions are concentrated, and among these, the Venezuela (Ven) accession also with its two individuals clearly separated. In this same group most of the accessions from Huila and Caldas appear dispersed and mixed among some of the ones from Valle del Cauca.
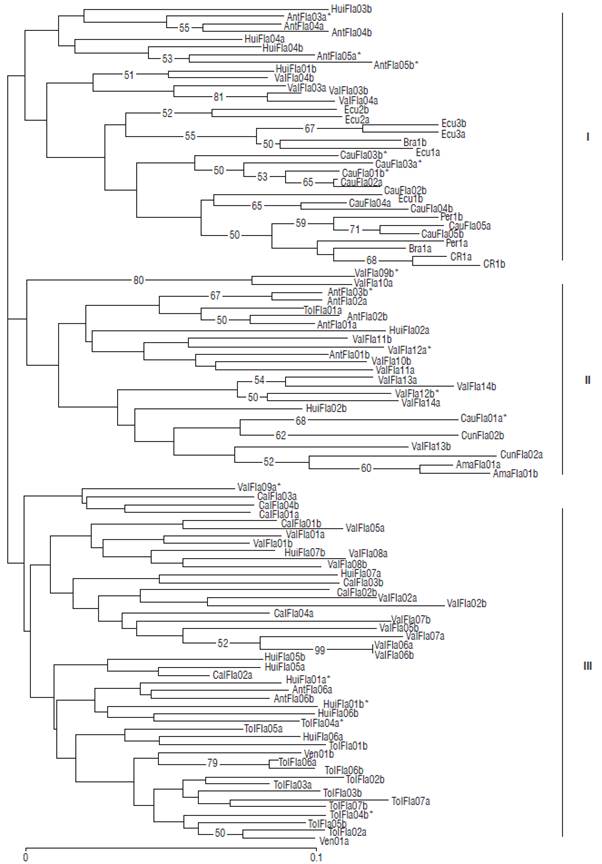
FIGURE 2 Dendrogram of 51 yellow passion fruit accessions constructed with the neighbor joining method and the coefficient of Nei (1978). Only bootstrap values greater than 50 are indicated inside the tree.
Genetic structure
The 58 alleles found in the 102 individuals were used to infer the genetic structure of the yellow passion fruit population. Structure analysis of the 51 accessions of P. edulis f. flavicarpa indicated K=4 as the uppermost hierarchical level of the genetic structure, while there were secondary peaks at K=8 (Fig. 3). Results of the Bayesian clustering indicated that the 102 individuals could be divided into four genetically distinct groups (Fig. 4). The first one (I) with 36 individuals is the largest group and there is an accession mixture of five different origins (Tolima, Huila, Antioquia and Caldas from Colombia, and Venezuela), with a clear grouping of the Tolima accessions (TolFla). The second group (II) brings together the accession from other countries (Costa Rica, Brazil, Peru and Ecuador) also includes the accessions from Cauca. The third group (III) is displayed similarly as the first one, but including the Amazonas, Antioquia, Caldas, Cundinamarca, Huila and Valle del Cauca accessions, indicating admixture between these populations of different geographic origin. The fourth (IV) and last group shows most of the accessions of Valle del Cauca clearly separated and related with some individuals of Huila and Caldas. The Bayesian clustering and the neighbor joining results are compatible, showing that almost all the yellow passion fruit accessions shared a similar genetic background.
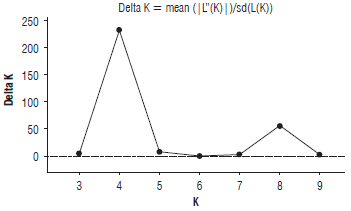
FIGURE 3 Delta K values compared to the number of groups (K) in the 102 yellow passion fruit individuals.
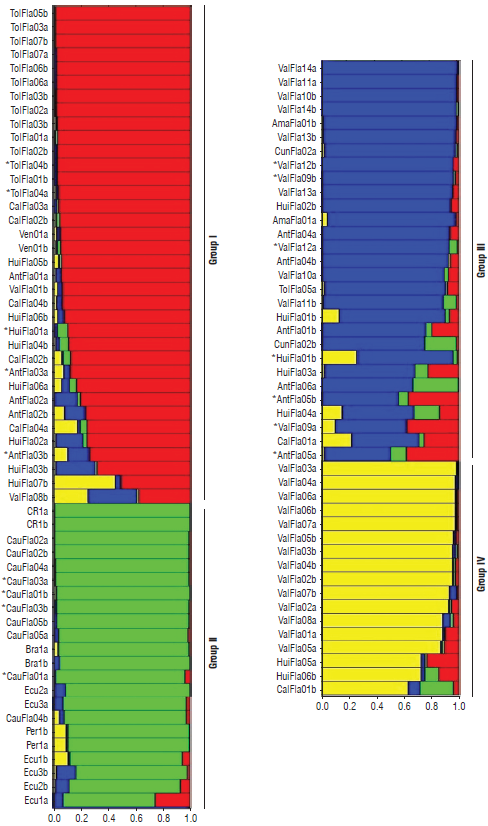
FIGURE 4 Bayesian analysis of the genetic structure (K=4) in the 51 yellow passion fruit accessions based on six microsatellite loci. Each bar represents one individual genotype, and individuals with multiple colors have admixed genotypes from multiple clusters. Elite accessions identified by Ocampo et al. (2013) are indicated with an asterisk (*).
In general, the microsatellite markers used allow the identification of a great genetic variability (He=0.78) in the yellow passion fruit accessions assessed, that moreover is reflected by differences between plants of each accession. Furthermore, the slight geographic structuring found may be the result of seed exchange between producers of different departments, and the type of cross reproduction found in yellow passion fruit.
Discussion
The polymorphic information content (PIC = 0.74 average) of most loci confirm that the evaluated markers are highly informative according to the criteria mentioned by Botstein et al. (1980), who consider that it must have a value that is higher than 0.50. This, because a higher PIC is related with the distribution and equilibrium of the population's allelic frequencies (Missio et al., 2010), that allows to assert that the selected markers are reliable and effective to detect genetic variability in the cultivated yellow passion fruit genotypes in Colombia. The effectiveness of the markers reached 60% in the yellow passion fruit germplasm evaluated in comparison to what Oliveira et al. (2005) reported. In the same way, in P. edulis f. edulis Sims (Ortiz et al., 2011) and P. ligularis (Bernal et al., 2014) the transferability of these microsatellites only reached 47 to 50%, suggesting that the transferability level could be associate to the taxonomic proximity of P. edulis f. flavicarpa with others species of genus Passiflora or the sample composition studied. Other studies conducted by Cerqueira et al. (2014b) and Silva et al. (2016) using a new set of microsatellites markers with Brazilian yellow passion fruit germplasm showed a low number of alleles (18 to 29, respectively) in comparison to our results (58 alleles). In contrast, Cerqueira et al. (2015) reported an elevated number of alleles (127) in 36 cultivated yellow passion fruit accessions in Brazil with 23 polymorphic microsatellite markers. However, in the later study the number of alleles is relatively low compared to the set of microsatellite markers evaluated.
Our results are contrasting with most of studies carried out in Brazil that mention a narrow to moderate genetic base of the commercial passion fruit cultivars available in Brazil. This may be due to the origin of the majority of the yellow passion fruit accessions characterized, as these originate from national institutional germplasm banks in Brazil, which could not be well represented genetically (Freitas et al., 2011).
Other differences between these results can be explained by the size and composition of each study sample and the degree of domestication present in each species or population. For instance, in sweet granadilla Bernal et al. (2014) report a considerable genetic variability (66 alleles, He=0.96) from Colombian germplasm. Nevertheless, the selection or domestication process in P. ligularis has been more recent, and just as other Andean species as Physalis peruviana L. and Solanum betaceum Cav., these have gone from being wild to cultivated species in just a few years (Pickersgill, 2007). Otherwise, the total (127) and private (31) number of alleles reported by Cerqueira et al. (2015) in Brazilian accessions can be the evidence of a higher intra-specific variability compared to the cultivated germplasm in Colombia, as Brazil is the primary center of diversity of yellow passion fruit. However, the low average number of microsatellite alleles is not always a consequence of the limited genetic variability, as it may depend on the sample size or on number of evaluated locus.
The average observed heterozygosity (Ho=0.52) was lower than the expected one (He=0.78) in most markers, suggesting an intermediate value of the heterozygotes observed in the population under Hardy-Weinberg equilibrium (HWE) conditions. This indicates that there is a slight endogamy tendency in the 102 individuals evaluated (Tab. 2) and within accessions of the same geographic or department origin (Tab. 3). This is probably due to the germplasm movement within Colombia, which comes mainly from nurseries located in the departments of Valle del Cauca (La Unión) and Huila (La Plata) towards others yellow passion fruit producing zones (Ocampo et al., 2013). Likewise, the yellow passion fruit producers generate their own seedlings, either from seeds collected in their neighborhood or from fresh fruits purchased at the market (pers. obs.). However, the average expected heterozygosity value (He=0.78) suggests that the yellow passion fruit germplasm evaluated possesses a considerable genetic variability in comparison with other studies carried out in Passiflora (Cerqueira et al., 2016). Furthermore, the presence of nine private alleles (10.3%) and a series of 31 alleles (58.6%) with low frequency (<10%) can be considered as a genetic reservoir for yellow passion fruit genotype selection, characterization and conservation processes for breeding programs. Likewise, this genetic reservoir of low frequency alleles could be a survival and adaptability mechanism to changing environmental conditions in natural surroundings where yellow passion fruit is cultivated, as the pathogen agent pressure or the climatic variability that is affecting world agriculture (Vermeulen et al., 2012). Additionally, an allelic richness parameter is a measure of genetic diversity that is commonly used in studies based on molecular markers that aim at selecting populations for conservation (Leberg 2002; van Zonneveld et al., 2102). Therefore, most of the accessions of Valle del Cauca due to their high genetic diversity (He=0.77), private alleles (5) and allelic richness (8 to 14 alleles) should be taken into account as a starting point for the use and conservation of genetic yellow passion fruit resources of in Colombia.
Genetic diversity and structure
The average value for the total genetic distance in the population was 0.683 and confirms a moderate genetic distance in the 51 yellow passion fruit accessions evaluated (Tab. 4). The tree classification shows three large groups (Fig. 1) with bootstrap values lower than 50%, indicating that the structuring of the groups found are not consolidated and can vary (Efron 1979). On the contrary, most internal branches are moderately supported (bootstrap ≥50%) and ratify the grouping between some accessions of the same department, e.g. ValFla, HuiFla y AntFla. The relationship between individuals of same accession is mostly low and does not show a geographical pattern according to the origin of each accession. In most cases, this unsteadiness occurs when some individuals at the moment they are grouped show intermediate similarity values compared to other groups, and therefore, are not assigned to a specific one and can belong to several comprised branches (Laurentin, 2009).
The microsatellite studied indicated that the population is structured in four main groups using Bayesian approaches but with great admixture among yellow passion fruit accessions of different geographic origins (Fig. 4). The possible causes of the failure of structure to define groups for 51 accessions according to its geographic origin is likely derived for the self-incompatibility of yellow passion fruit and its outcrossing pollination. Regarding the elite accessions identified by Ocampo et al. (2013) and evaluated in this study, these can be found spread in different groups (AntFla03, 05; CauFla01, 03; HuiFla01; TolFla04 and Val-Fla09, 12). These accessions are favorable scheme to select parental materials with promising and desirable agronomical traits for hybridization in yellow passion fruit breeding programs in Colombia.
The set of results of cluster and structure analyses show that the accessions analyzed are genetically heterogeneous and indicate that there is low correspondence with geographic distribution of accessions, and this is similar to other studies reported for cultivated accessions of P. ligularis (Bernal et al., 2014) and Carica papaya (Matos et al., 2013). In contrast, Ortiz et al. (2012) reported a high genetic homogeneity in cultivated material of P. edulis f. edulis (purple passion fruit) in Colombia with microsatellite markers and AFLP, and without a consistency with the origin of the samples analyzed by department or location. Nevertheless, the cultivated yellow passion fruit accessions in Colombia mentioned above have not been subject to an adequate selection plan, as farmers inconsistently choose the best fruits in each harvest, without considering cross pollination (Ocampo et al., 2013). This type of phenotypic selection only allows knowing the attributes of mother plants, as seeds of harvested fruits constitute families of half siblings. In consequence, the crops show a genetic diversity degeneration in terms of structuring, and indirectly greater susceptibility to phytosanitary problems such as trips (Neohydatotrhrips signifer), ovary flies (Dasiops inedulis), fungi (Fusarium oxysporum) and viruses (SMV and PSLDV).
These analyses showed that there is high genetic variability in the yellow passion fruit accessions cultivated of Colombia. The majority of the Colombian accessions show dissimilar genetic backgrounds and are likely derived from a large number of introductions that occurred from Hawaii (USA) and Brazil from the 1960's. Moreover, this is also probable due to the gene flow via seeds movement among farmers of different geographic origins and to the self-incompatibility allogamy of this species, resulting in an increase in allele distribution among different accessions. This is consistent with the results found by various authors in other fruit trees as Carica papaya L. (Ocampo et al., 2007; Matos et al., 2013), P. ligularis (Bernal et al., 2014) and Physalisperuviana L. (Chacón et al., 2016) who argued that genetic polymorphism can be associated with the al-logamous nature of the species and the exchange of seeds between producers, which tends to favor the conservation of a high percentage of heterozygote genotypes.
Our study illustrates the usefulness of using microsatellites to carry out genetic analyses within a species with high polymorphism levels as the case of yellow passion fruit. The analysis of the genetic structure of the populations considered here could be extremely useful, as the information obtained helps us to get the knowledge on the species germplasm to better use these genetic resources in search of new planting material for commercial plantations in Colombia.
Breeding implications
This study establishes a considerable variability (He=0.78) in the cultivated yellow passion fruit materials considered in this research in Colombia, which have been structured in four main subgroups. This information is of utmost importance for plant breeders as it shows and establishes a genetic structure and variability in cultivated yellow passion fruit, as a base for the search of genotypes that are more productive and resistant to phytosanitary problems (Faleiro et al., 2001; Cerqueira et al., 2016). The establishment of the genetic structure of the populations settled out in this study showed a defined assessment of the genetic diversity among 51 yellow passion fruit accessions. Yellow passion fruit breeding programs will benefit and will have a guide to develop their genetic breeding strategy by the convergence not only of the above mentioned information, but also with the results obtained from the evaluation of this material in the field, and by carrying out designed crosses between accessions belonging to different groups to maximize and attain significant variation of their offspring. Likewise, it is also necessary to complement the molecular marker information with morphological data of each accession that will allow the establishing of the true relationship between phenotypic and genotypic variations as has been reported in other successful studies (Reis et al., 2012). Moreover, the accessions with certain fruit quality characteristics are sources of clones and should be proposed as base for superior genotype selection to get earlier-ripening and more productive cultivars (Silva et al., 2016), starting from selective processes in local populations and focusing on direct farmer participation. Still, the starting point in the breeding strategy of the yellow passion fruit in Colombia are the elite accessions identified by Ocampo et al. (2013), as these comply with physicochemical parameters required by the market as fruit weight of ≥200 g, pulp percentage of ≥50% and °Brix of≥14.5 (Tab. 1). However, these accessions must be evaluated in the field at different selection cycles to establish interesting characters with controlled pollinations to avoid gene flow with unwanted genotypes and through a multivariate analysis. Additionally, it is important to include those individuals that possess private (Valle del Cauca, Antioquia, Huila and Tolima) and rare alleles to enrich the primary gene pool of the cultivated accessions as a strategy to conserve and improve the genetic resources of the species. The above mentioned will also allow the marker-assisted selection (MAS) of parentals with a wide quantitative genetic distance for the development of per se hybrids with high heterosis in the F1 generation. Finally, this is the first study of genetic diversity in cultivated yellow passion fruit in Colombia using microsatellite markers, and it proposes this referenced technique as an effective tool to characterize species germplasm, but must be completed with agromorphological data or Genotyping by Sequencing (GBS) studies that will allow the establishment of the total variability degree. The considerable variability found in the Colombian cultivated yellow passion fruit materials assessed in this survey and that were structured in four main subgroups, could be considered as important information that can further be used to direct future population crosses in breeding programs, leading the selection of interest traits while maximizing heterosis. If this is not maintained it could generate a severe long-term inbreeding and the genetic variability decrease.
Conclusions
We conclude that the use of microsatellite markers was highly informative for the 51 yellow passion fruit accessions (average P7C=0.74), presenting a high average number of alleles per locus (9.66). This study found important levels of variability with the disclosure of alleles of low frequency (40 alleles) and elevated expected heterozygosity (He=0.78), but with low genetic differentiation among different origin accessions. Moreover, the genetic diversity and the population structure revealed by some Colombian yellow passion fruit accessions considered, provided information that can be useful to make strategic decisions regarding potential crosses in current passion fruit breeding programs of commercial type in the country. Additionally, accessions from Valle del Cauca concentrate most of the allelic richness (11 to 14) and should be considered when establishing in situ, on-farm or ex situ conservation strategies in Gene Banks.