Introduction
The family Heliconiaceae consists of 182 neotropical species distributed in Central and South America. Twenty-nine species are found in Brazil, five of which are endemic (Braga, 2014). Heliconiaceae are distributed in the Central-West, Northern, Northeastern, and Southeastern regions of the country. Nine species of the genus inhabit the state of Mato Grosso, i.e., Heliconiapsittacorum, H. rostrata, H. episcopalis, H. marginata, H. subulata, H. acuminate, H. hirsuta, H. densiflora, and H. stricta; the last two were first recorded in Mato Grosso (Braga, 2014).
The state of Mato Grosso lies in the so-called Ecological Transition Zone (ETZ) between the biomes savanna (Cerrado) and rainforest (Amazon region). The ETZ is a complex morphoclimatic domain at the North of the Cerrado and Southwest of the Amazon, where savannas and tropical forests coexist under similar environmental conditions (Furley et al., 1992).
Heliconiaceae species have herbaceous plants used in floriculture as ornamental plants, grown under full sun or partial shade, or as cut flowers, because the terminal inflorescences with intense colors of different shapes and sizes are greatly appreciated for event decoration and floral arrangements. Only few and recent studies addressed the agronomic potential of ornamental interest species. In the forest region Zona da Mata of Pernambuco, some studies analyzed genetic parameters of seven genotypes of H. psittacorum (Costa, 2007; Rocha et al., 2010; Araujo et al., 2015).
Due to the large natural variability in Heliconia sp. populations and the potential of these species as ornamental, research on breeding, agronomic and genetic characterization should be intensified. A study target of breeding programs is to collect accessions in genebanks to develop genotypes with traits of economic interest that meet the demands of the ornamental market (Rocha et al., 2010). Moreover, the genetic divergence and parameters can be analyzed to estimate genetic gains and determine the most appropriate breeding method (Cruz et al., 2012).
This study addressed the morphological description and the estimation of genetic parameters and divergence of H. psittacorum and H. densiflora accessions, with a view to evaluate the genetic variability in the state of Mato Grosso, identifying possible parents to breed future hybrids with ornamental potential.
Material and methods
The germplasm collection of Heliconias (BAG) of the State University of Mato Grosso (UNEMAT) was established in March 2014, in an experimental field in the municipality of Tangará da Serra, MT (14°39' S and 57°25' W; elevation 321 m a.s.l.). The climate is tropical, with well-defined dry and rainy seasons and annual means varying from 1,300 to 2,000 mm rainfall and 16-36°C temperature range (Martins et al., 2010). The soil, with a flat to slightly wavy relief, is classified as Latossolo vermelho distrófico (Embrapa, 2006).
The area for planting of seedlings was harrowed and limed according to soil analysis. Fertilization at planting consisted of 50 g monoamonic phosphate (MAP) per planting hole and topdressing of monthly applications of 50 g urea and 20 g potassium chloride per hole and of 50g MAP every 6 months.
Four accessions of H. densiflora and 14 of H. psittacorum, from 13 municipalities in the state of Mato Grosso, were described (Tab. 1). The clumps were divided and four rhizomes planted, at a spacing of 3.0 m between rows and 1.5 m between plants in full sun. When needed, irrigation was applied three times a week. The experiment was conducted as described by Costa et al. (2007), in a randomized block design with 18 treatments (accessions), four blocks (replications) with one rhizome per plot.
TABLE 1 Accessions of Heliconia densiflora and H. psittacorum collected in different municipalities in the state of Mato Grosso, Brazil.
| Accession | Species | Municipality | Latitude | Longitude | Altitude m a.s.l. |
|---|---|---|---|---|---|
| 1 | H. densiflora | Alta Floresta | 9°51'05" | 56°12'31 " | 281 |
| 2 | H. densiflora | Alta Floresta | 9°51'47" | 56°12'04" | 271 |
| 3 | H. densiflora | Alta Floresta | 9°52'43" | 56°9'22" | 281 |
| 4 | H. densiflora | Carlinda | 10°10'8" | 55°48'53" | 299 |
| 5 | H. psittacorum | Nova Canaã | 10°3644" | 55°42'05" | 265 |
| 6 | H. psittacorum | Colíder | 10°46'55" | 55°27'00" | 310 |
| 7 | H. psittacorum | Matupá | 10°12'26" | 54°57'39" | 260 |
| 8 | H. psittacorum | Guarantã Norte | 9°46'02" | 54°53'55" | 348 |
| 9 | H. psittacorum | Guarantã Norte | 9°44'26" | 54°53'16" | 336 |
| 10 | H. psittacorum | Peixoto Azevedo | 10°16'9" | 55°01'15" | 324 |
| 11 | H. psittacorum | Terra Nova do Norte | 10°44'5" | 55°08'43" | 295 |
| 12 | H. psittacorum | Santo Afonso | 14°35'9" | 57°10'56" | 494 |
| 13 | H. psittacorum | Nova Marilândia | 14°21'5" | 57°02'01" | 355 |
| 14 | H. psittacorum | Tangará da Serra | 14°42'2" | 57°47'31" | 204 |
| 15 | H. psittacorum | Barra do Bugres | 15°07'6" | 57°04'34" | 156 |
| 16 | H. psittacorum | Porto Estrela | 15°18'1" | 57°10'11" | 168 |
| 17 | H. psittacorum | Porto Estrela | 15°24'2" | 57°11'51" | 148 |
| 18 | H. psittacorum | Porto Estrela | 15°35'37" | 57°11'51" | 155 |
Qualitative and quantitative morphological traits were evaluated around 400 d after planting, when the plants were fully established. Twenty-five descriptors were used, of which 15 were quantitative and 10 qualitative. The quantitative descriptors were: LL (cm) leaf length; LW (cm) leaf width; NLS (n) number of leaves on the flower stem; NIC (n) number of inflorescences per clump; SW (g) shoot weight (leaves and floral stem); FSW (g) flower stem weight without leaves, flower peduncle and inflorescence; FSL (cm) flower stem length; FSD (cm) flower stem diameter, measured 20 cm below the inflorescence; IL (cm) inflorescence length; LI (cm) inflorescence width; NFI (n) number of flowers per inflorescence; NBI (n) number of bracts per inflorescence; BL (cm) bract length; BD (cm) bract depth; and SL (days) longevity of the flower stem. The qualitative descriptors evaluated in the inflorescences and leaves were: inflorescence growth, hairiness, waxiness, bract and flower color; arrangement of bracts (flat or spiral); hairiness and waxiness of the flower stem (pseudostem + floral peduncle + inflorescence) and leaves; firmness of the flower stem (bracts + rachis), (Costa et al. 2007).
Flower stems with two to three open bracts were cut between 7:00 and 8:00 h, twice a week, for 1 month. The stalks were stored in water recipients and transported in buckets to the postharvest laboratory. The inflorescences were cleaned (removing the flowers from within the bracts), washed, and cut to a standardized stem length of 80 cm). Quantitative and qualitative traits of five flower stems per clump were assessed, with four replications.
The flower stalks were placed in containers with water, which was exchanged every 2 d and maintained at 19°C (cold room). The postharvest shelf-life (days) was evaluated every 2 d, for 21 d and the stems discarded when the bracts darkened on the inflorescences.
For the quantitative descriptors, analysis of variance was performed, the Scott Knott grouping test was applied and genetic parameters associated with efects of genetic and environmental nature, were estimated according to Cruz et al. (2012). The values of the genotypic determination coefficient (H2) were expressed as the proportion of phenotypic variance caused by the genetic variability among treatment means (Cruz, 2006), and it can be used when the effect of genotypes is fixed, as in the case of this study.
To quantify the genetic divergence among accessions, Mahalanobis' generalized distance (D2) was used for quantitative variables, while the simple match distance was used for the qualitative descriptors. The relative importance of variables was also evaluated by the methodology of Singh (1981). For the simultaneous analysis of qualitative and quantitative variables, the Gower's General Coefficient of Similarity was performed (1971).
The statistical analysis was performed using GENES software (Cruz, 2014). The accessions were grouped by the hierarchical method Unweighted Pair-Group Method Using Arithmetic Averages (UPGMA) and validated by the cophenetic correlation coefficient using the software MEGA, version 5 (Kumar et al., 2009). The Pearson correlation between the distance matrices was also performed and the significance determined by the Mantel test (10,000 permutations). All Mantel tests performed here were conducted using the R packages vegan (Oksanen et al., 2012).
Results and Discussion
All H2 values exceeded 87%, except for NIC (54.28%) and NFI (67.55%), which were considered low (Tab. 2). For the H2 values, the variation index (Iv) was considered. According to Vencovsky and Barriga (1992), an Iv higher than one (1.0) indicates good conditions for selection gains by simple breeding methods, such as mass selection.
TABLE 2 Estimates of the genetic parameters of four accessions of Heliconia densiflora and 14 accessions of H. psittacorum.
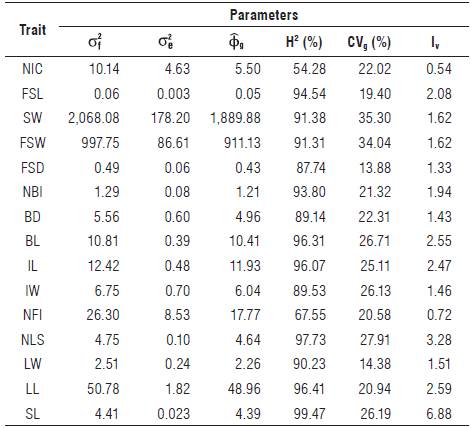
NIC: number of inflorescences per plant; FSL: flower stem length; SW: flower stem weight with leaf; FSW: fresh stem weight without leaf; FSD: diameter of the flower stem; NBI: number of bracts per inflorescence; BD: bract depth; BL: bract length; IL: inflorescence length; IW: inflorescence width; NFI: number of flowers per inflorescence; NLS: number of leaves on the flower stem; LW: leaf width; LL: leaf length (LL); SL: stem longevity.
The selection gain for the traits NIC and NFI may be minimized, since the H2 value is low and Iv <1.0. The results also suggest the existence of genetic variability among accessions of Heliconia sp. for the studied traits, indicating favorable genetic values for breeding programs. For the 15 evaluated quantitative traits, several morphological groups were identified (Tab. 3)
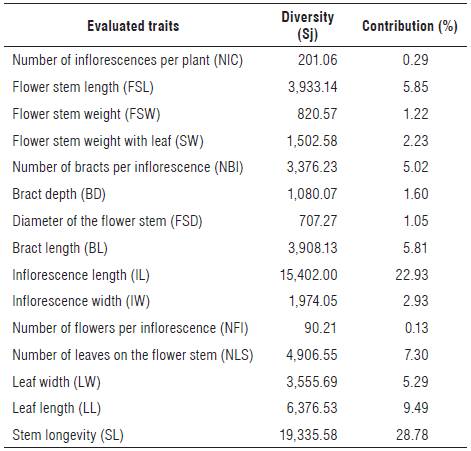
TABLE 3 Relative contribution of 15 quantitative traits to genetic variability detection, based on Mahalanobis' generalized distance between 18 accessions of Heliconia densiflora and H. psittacorum.
Analyzing agronomic traits of H. psittacorum genotypes under full sun and partial shade, Costa et al. (2007) found H2 varying from 82.25 to 97.33%, except for the trait days to inflorescence cut (20.02%). Similar results were reported by Rocha et al. (2010), in a study with H. psittacorum cultivars and interspecific hybrids, where Iv values between 0.21 and 1.85 were recorded for seven traits (days until inflorescence sprouting, period until stem harvest, cycle, stem weight without leaves, stem diameter, inflorescence length, number of open bracts per inflorescence).
The inflorescence traits such as length, stem length, fresh weight, and durability are of great commercial interest, due to promising ornamental potential. Inflorescences of Heliconiaceae can be classified in small (up to 10 cm), medium (10.1 to 30 cm), large (30.1 to 50 cm), and very large (>50 cm) (Castro et al., 2007). Of the 18 accessions, 16 were classified as medium and two (accessions 5 and 16) as small (Tab. 4). The small to medium inflorescences are less difficult to handle and transport (Castro et al., 2007). Genetic diversity studies of H. psittacorum and interspecific hybrids in the state of Pernambuco registered inflorescence lengths from 12.1 to 23.3 cm (Rocha et al., 2010).
TABLE 4 Mean values of 15 quantitative traits of Heliconia densiflora (accessions 1-4) and H. psittacorum (accessions 5-18).
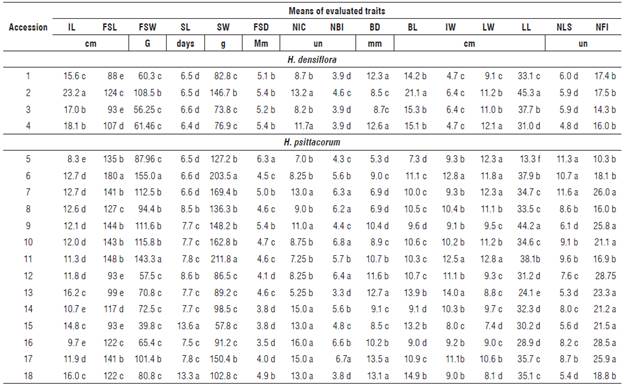
IL: inflorescence length; FSL: flower stem length; FSW: fresh stem weight without leaf; SL: stem longevity; SW: flower stem weight with leaf; FSD: diameter of the flower stem; NIC: number of inflorescences per plant; NBI: number of bracts per inflorescence; BD: bract depth; BL: bract length; IW: inflorescence width; LW: leaf width; LL: leaf length; NLS: number of leaves on the flower stem; NFI: number of flowers per inflorescence.
Means followed by the same letter in the column do not differ statistically by the Scott-Knott test at 5% probability.
The traits FSL and FSD are important to ensure the quality and success of Heliconia species on the market. As standard length of floral stems of Heliconiaceae,Loges et al. (2005) suggested 80 cm. On the other hand, the higher the FSL, the greater the risk of stem breaking. Consequently, accessions with a FSL equal to or greater than 80 cm are recommended for breeding programs (Tab. 4).
The fresh weight of flower stems without leaf (FSW) ranged from 39.8 to 155.0 g (Tab. 4). This trait directly affects the stages management, preparation, packaging, and transport. The success of cut flowers on the market depends on the selection of accessions with a stem durability of more than 10 d. Long-lived stems can reach markets that are more distant by extending the shelf-life and maintaining the commercial quality (Castro et al., 2007).
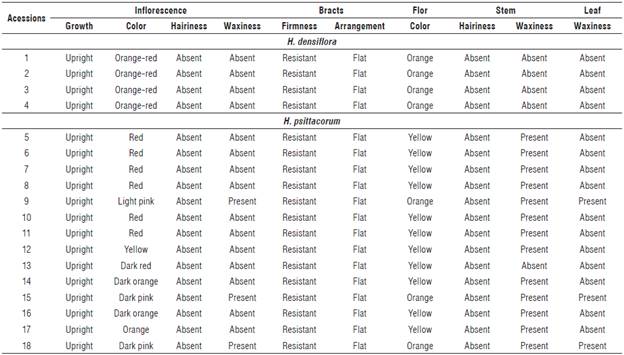
In the assessment of genetic diversity based on the 15 quantitative traits, three groups were determined using 255 as a cut distance (Fig. 1). Group I comprised the accessions 1, 2, 3, 4, 6, 8, 9, 10, 11, 12, 13, 14, 16, and 17, Group II accession 5 and Group III accessions 15 and 18. The isolation of the acession 5 of H. psittacorum in Group II was based on the traits of FSD and LL (Tab.4). The FSD expresses greater resistance of the flower stem against breaking by wind in the field, to transportation from the field to the location of cleaning, and in the subsequent selection stages (Albuquerque et al., 2010).
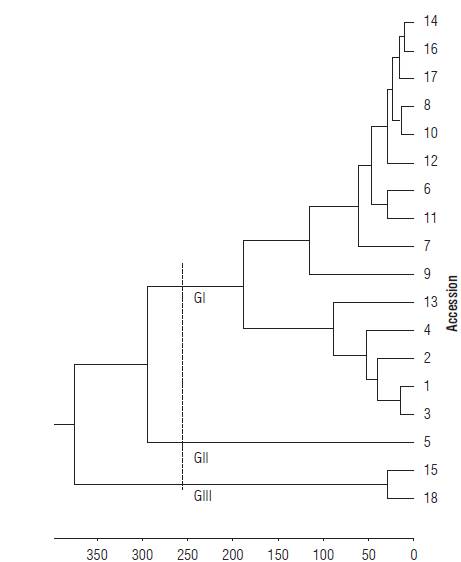
FIGURE 1 Dendrogram of genetic dissimilarity between Heliconia densiflora (accessions 1-4) and H. psittacorum (accessions 5-18), obtained by the UPGMA method, based on 15 quantitative traits, using Mahalanobis' generalized. Cutting distance 255 (Cophenetic correlation: 0.87; Distortion: 8.3%; Stress: 28.9%).
The cophenetic correlation for the data was 0.87, confirming the reliability of the conclusions based on the visual assessment of the dendrogram, since values above 0.7 indicate a good adjustment of the dissimilarity matrix and the graphical representation of the distances (Sokal and Rohlfe, 1962). The accessions of the species H. densiflora (accessions 1, 2, 3, and 4) were grouped in the same group (G I), while the accessions of the species H. psittacorum were separated in two groups.
The highest contributions to the determination of the divergence of the studied accessions (Tab. 4) were related to the traits longevity of the flower stem (SL) with 28.78%, and inflorescence length (IL) with 22.93%, both accounting for 51.71% of the variation among accessions. Stem durability was an important trait for the genetic divergence among accessions, since the accessions 15 and 18 with highest SL formed one group (Group III). On the other hand, the flower stem diameter (FSD) and leaf length (LL) forcing the isolation of accession 5, are traits with no significant contribution to the genetic divergence among accessions (Tab. 4; Fig. 1)
To ensure the success in parental selection for breeding programs, less relevant traits should not be considered in characterization and evaluation studies and the other traits with high discriminatory potential maintained (Cruz et al., 2012). The traits that contributed least to divergence were number of flower inflorescences (NFI) (relative contribution to variation 0.13%) and number of inflorescences per clump (contribution 0.29%) (Tab. 4), which were therefore discarded.
Among the 10 qualitative traits evaluated, six indicated no polymorphism among accessions, e.g.: upright inflorescence position, absence of hairiness on flower stem and leaves, and firmness and flat arrangement of the bracts. Moreover, the accessions differed in terms of flower and inflorescence colors and presence of waxiness on the inflorescence, leaves and stem (Tab. 5).
TABLE 5 Qualitative traits evaluated in 18 Heliconia sp. accessions, collected in municipalities of the state of Mato Grosso, Brazil.
Tropical flowers differ from other more traditional plants on the market in their diversity of shapes, resistance to transport and durability (Loges et al., 2005), aside from attractive visual aspects, being widely used for floral arrangements, event and party decor, cultivation in gardens, and projects of ornamental garden design. In studies of Heliconia sp species focused on cut flowers, Castro et al. (2007) reports that the high contrast of bract colors is one of the traits responsible for the appeal of this ornamental plant on the market. In the accessions, eight colors were identified in the inflorescences and two in the flowers (Tab. 5). Thus, the existence of variability for the traits in flower and inflorescence color is fundamental for successful breeding.
Waxiness on inflorescences and leaves was detected in 21.43% of the accessions and 92.85% of the floral stems of H. psittacorum accessions (Tab. 5). Waxiness is an undesirable trait, since the visual aspect can be affected by handling (Loges et al., 2005). This trait was not observed in H. densiflora accessions (Tab. 5).
Considering only the qualitative traits, two groups were determined using 0.148 as a cut distance. Group II contained the accessions 9, 15 and 18 and Group I the remaining accessions (Fig. 2). The cophenetic correlation was 0.94, also considered high. No polymorphism was detected for accessions of the species H. densiflora (accessions 1, 2, 3, and 4). Once again, the accessions of species H. psittacorum were classified in two distinct groups, with polymorphism within the species.
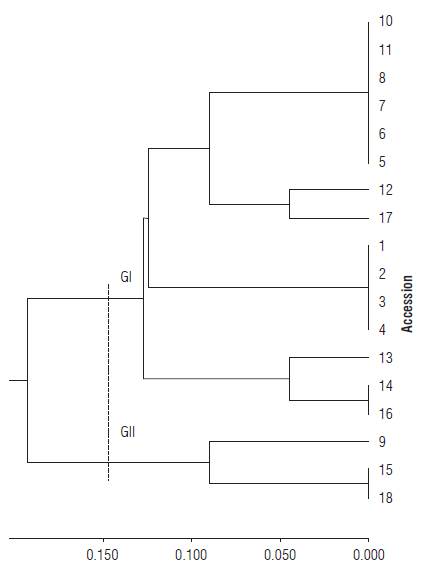
FIGURE 2 Dendrogram of genetic dissimilarity between Heliconia den-siflora (accessions 1 to 4) and H. psittacorum (accessions 5 to 18), based on the UPGMA method, using 11 qualitative traits and the Simple Match. Cutting distance 0.148 (Cophenetic correlation: 0.94. Distortion: 2.7%. Stress: 16.4%).
The correlations between dissimilarity matrices quantitative, estimated by Mahalanobis (0.74), qualitative Simple Match (0.62) and combined Gower distance (0.84) the whole set of traits, were all significant and high.
In the combined analysis (Gower General Coefficient of Similarity), showing correlation between the matrices of original distances and the cluster matrix. Using 3.6 as cut distance three groups were determined: Group I contained the accessions 5, 6, 7, 8, 10, 11, 12, 13, 14, 16, and 17, Group II accessions 1, 2, 3, and 4, and Group III accessions 9, 15 and 18 (Fig. 3). The H. psittacorum accessions were assigned to Group I and III. The H. densiflora accessions were clustered in Group II, showing the low variability of these accessions, probably due to the low number of accessions.
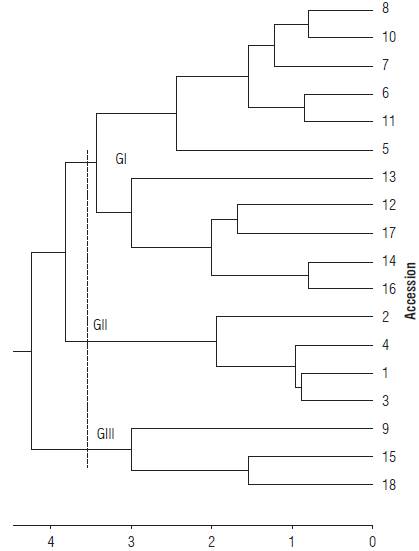
FIGURE 3 Dendrogram of genetic dissimilarity between Heliconia den-siflora (accessions 1 to 4) and H. psittacorum (accessions 5 to 18), obtained by the method UPGMA, based on 15 quantitative and 11 qualitative traits, using the Gower distance. (Cophenetic correlation: 0.84. Distortion: 2.9%. Stress: 17.0%). Cutting distance 3.6.
In Group I, accession 13 stood out with a lower FSL and FSW, along with the absence of waxiness on the inflorescence, stem and leaf. In addition, the inflorescences are dark red and the flowers yellow.
In Group II, the accessions 1, 2 and 4 had low FSL and FSW values and no waxiness on the inflorescence, stem and leaf, as well as highest FSD and orange-red inflorescences and orange flowers.
In Group III, accessions 15 and 18 stood out for good postharvest shelf-life. In breeding programs, parents with high genetic divergence are recommended, to promote the occurrence of superior segregating plants in subsequent generations. However, the choice of individuals with superior plant, flower and fruit traits is also important, and finally, performance analysis for later recommendation of use per se. Thus, crosses should be performed between different genotypes with desirable agronomic traits (Cruz et al., 2012).
To this end, we suggest to cross accessions 15 and 18 (Group III) with accessions 1, 2 and 4 of Group II and/or accession 13 of Group I, to breed superior genotypes.
Studies of Gomes et al. (2016) pointed out that among Heliconias species, H. psittacorum cultivars and hybrids have a number of interesting traits, such as year-round production, terminal and upright inflorescences, varied bract number, and different types of flower colors, as observed in this study.
In this sense, based on the traits evaluated, the studied accessions are of interest for ornamental use, enabling a diversification of the ornamentals market. Traits such as bract length are relevant for playing an important role in the composition of floral arrangements and to raise the interest of consumers, as was expressed by Albuquerque et al. (2010).
The diameter and length of the flower stem are also key traits to characterize Heliconia sp. accessions. They make the flower stem more resistant to winds, to the transport from the field to the location of cleaning and the selection steps, and extend the postharvest shelf-life.
Conclusions
Morphological characterization of Heliconia species allowed the recognition of those descriptors which contribute to the detection of genetic divergence and provided key knowledge of the patterns of variation phenotypic of the target species.
The genetic variability among accessions of germplasm collection of Heliconia sp. uncovered in the present study can be explored in the breeding programs.
The characteristics that contributed most to the detection of genetic divergence were floral stem durability and inflorescence length.














