Introduction
Many American indigenous bees pollinate native and domesticated plant species, in some cases even, more effective and efficiently than the imported bee Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae). According to Klein et al. (2007) from 57 crops accounting the 95% of the world production, 42% are visited by a native bee.
Among stingless bees those of the tribe Meliponini stand out, they do not sting because the sting is atrophied. They are distributed throughout the tropical and subtropical region of America, Africa, Asia and Oceania (Michener, 2002; Nogueira-Neto, 1997). In Latin America the exploitation of honey and wax from meliponines or the so called meli-poniculture, a term created by Nogueira-Neto. (1997), has been reported before the arrival of Columbus (McGregor, 1976). In Colombia, few documents about this culture exist, however the use of 25 species has been recorded (Nates-Parra and Rosso-Londoño, 2013). In Brazil, until the introduction of the domestic bee in 1839, they were the only producers of honey (Witter and Tirelli, 2014), while in Colombia they were of great importance to the development of native communities, long before the conquest. The native tribes Chibchas, Muiscas and Tayronas, among others, consumed their honey to sweeten the "chicha" and used its wax in gold melting for jewelry production, using the lost wax technique (Falchetti and Nates-Parra, 2002, Nates-Parra, 1996).
Meliponines are social insects that offer a great service by effectively pollinating native and cultivated plants (Witter and Tirelli, 2014). Bees of the genus Scaptotrigona and Tetragonisca can be used to pollinate field and greenhouse crops, due to their docility, absence of functional sting and adaptation to artificial domains (McGregor, 1976; Nates-Parra, 2005).
Tetragonisca angustula Illiger, 1806, according to Nogueira-Neto (1997) is one of the bees with very good characteristics to "meliponiculture", both to honey production and pollination. Workers can cover a distance of 500 m, are generalist and polylectic, which means they can visit flowers of various plant species and are rustic (Nates-Parra, 2005; Venturieri, 2008). In Colombia, the species has been recorded from 0 to 2,000 m a.s.l. in most of the national territory (Nates-Parra and Rosso-Londoño, 2013).
Scaptotrigona xanthotricha Moure, 1950 called in Brazil "mandaguari-amarillo", covers a distance of approximately 750 m (Nogueira-Neto, 1997); its behavior is more aggressive than T. angustula. They do not possess a functional sting, but defend their hive by tangling in operator's hair and clinging with legs and mandibles to the cloth and sensitive skin areas like eyelids and lips, without causing any physical damage. They show great potential as a producer species of special honey with antimicrobial activity (Borsato et al., 2013); distributed in several states in Brazil (Camargo and Pedro, 2008) and registered in Colombia in the department of Santander (Nates-Parra, 2001).
Although there is a considerable number of articles over the toxicology and the effect of insecticides on meliponines, as those published by Costa et al. (2015), Jacob et al. (2013), Lourenço et al. (2005), Moraes et al. (2000), Soares et al. (2015), Tomé et al. (2015), Valdovinos-Nuñez et al. (2009), among others, there is few information on the toxicology of the two species mentioned above and even less about neonicotinoids and phenylpyrazoles based insecticides, such as thiamethozam and fipronil.
Thiamethoxam belongs to the neonicotinoids, agonistic molecules of the nicotinic acetylcholine receptor, that mimic the action of the neurotransmitter acetylcholine, blocking receptors and disrupting the transmission impulse between nerve cells (Devine et al., 2008, Group IRAC International MoA Working, 2015); it is systemic and acts by ingestion and contact, as well as its metabolite clothtianidin. Both compounds are persistent in soil and water and are highly toxic to Apis mellifera (Lewis et al., 2016). Nowadays, Collapse Colony Disorder (CCD) is one of the most important problems that threaten A. mellifera and, consequently, agriculture in North America and Europe. CCD is defined as: "the loss of bee colonies without the presence of dead adult bees; only the queen is found, sometimes honey and immature bees" (USDA ARS, 2008). As other insecticides belonging to this group, the use of thiamethoxam is one of the possible causes of this disorder, both directly and indirectly, by increasing the susceptibility of bees to pathogens such as Nosema that affects the digestive tract of bees (CCD Steering Committee, 2012, Henry et al., 2012). Due to these results, several countries of the European Union have banned and / or limited its use in several crops (Agriculture forêt, 2013).
Fipronil belonging to phenylpyrazoles, it is also a neurotoxic antagonist of the chloride channel regulated by γ-aminobutyric acid (GABA); due to its mode of action interferes with the chloride channels in the nerve membrane, disrupting ion transfer and impulse transmission between nerve cells (Devine et al., 2008, Group IRAC International MoA Working, 2015). It is systemic and can act by ingestion and contact; persistent in soil as well as its most important metabolites, product of its transformation, fipronil amide, sulfone and sulphide, of which the toxicity to bees has been only studied for fipronil sulfone, product, orally, highly toxic (Lewis et al., 2016). It is reported as a toxic compound for bees and also as a possible, partial, CCD responsible (Reuber, 2015); in addition, causes a greater susceptibility of bees to Nosema (Vidau et al., 2011).
In Colombia there are two products registered with thiamethoxam as active ingredient and two in mixture with lambda-cyhalothrin; for fipronil more than 50 products with the active ingredient are registered alone and in mixture. Its use is permitted in rice, maize, potato, pasture, tomato, bean, citrus, onion, soybean, green beans, chrysanthemums, rose, avocado and cotton (ICA Instituto Colombiano Agropecuario, 2016).
In order to contribute to the knowledge of the toxicology of these two products commonly used in agriculture and to formulate strategies to increase productivity, toxicity and the effect of thiamethoxam and fipronil on T. angustula and S. xanthotricha was evaluated, estimating the LD50 applied both topically and orally.
Materials and methods
The research was carried out at the phytosanitary laboratory of the Agronomy Faculty Eliceu Maciel, Federal University of Pelotas, Capão do Leão Brazil, where artificial hives of Scaptotrigona xanthotricha and Tetragonisca angustula (Hymenoptera: Apidae) are available.
To perform the tests, the methodologies proposed in the Guide 213 Honeybees, Acute oral Toxicity Test and Guide 214 Acute Contact Toxicity Test of the Organization for Co-operation and Economic Development (OECD/OCDE, 1998); OCSPP 850.3020 : Honeybee Acute Contact Toxicity Test and OCSPP 850.3030: Honey Bee Toxicity of Residues on Foliage, The United States Environmental Protection Agency (EPA, 2012a, 2012b), the Guideline for Evaluating Side Effects of Plant Protection Products on Honeybees from The European and Mediterranean Plant Protection Organization (OEPP/EPPO, 2010) and the Standard Methods for Toxicology Research in A. mellifera of the International Bee Research Association (Medrzycki et al., 2013) were considered.
Collection of bees: The collection was made with an aspirator, lifting the top of the hives. Initially, 180 bees per test, ten bees for three replicates and six treatments were collected, then deposited in a plastic container with a lid and a feeder with a 50% sucrose solution (EPA, 2012a, 2012b; Medrzycki et al., 2013, OECD/OCDE, 1998; OEPP/EPPO, 2010). The bees were kept for 24 h in a dark room at 26°C and 70% humidity (EPA, 2012b; OECD/ OCDE, 1998; OEPP/EPPO, 2010).
Specimen conditioning prior to application: The importance of food availability at the collection event has been observed. Maintaining these meliponines for more than 30 minutes without food causes a high natural mortality in the vials. For A. mellifera it is not recommended to have an individual alone for more than 1 h (OEPP/EPPO, 2010); since the meliponines are also social insects, this recommendation was considered.
The individuals were removed from the darkroom and all dead bees or those that did not exhibit normal and active behavior were discarded. The normal ones were anesthetized with CO2 for 20 s or until sleeping.
Preliminary tests were performed to evaluate the response of individuals to the anesthesia procedure, to determine the volume of solution to be applied to the individual for topical application, and to know the effect of solvents for both topical application and oral exposure.
After the application of the chemical the individuals were transferred to an aluminum box with two holes and metal mesh where they had a 50% sucrose based feeder; all 18 experimental units corresponding to each test were taken back to the dark room.
Insecticide Solutions: The dilutions were prepared according to the commercial formulated products guidelines: Actara 250 WG containing 25% m/v of thiamethoxam and Standak® containing 25% m/v of fipronil. As base solution the maximum recommended dose for field applications (MDRC) of the insecticides was taken, prepared with water and 1% detergent due to the insolubility of both commercial products in acetone. Serial dilutions were prepared from the base solution until the required doses for topical application and oral exposure were reached (Medrzycki et al., 2013).
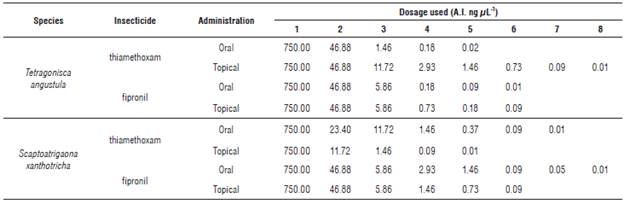
The doses used (Tab. 1) varied according to the behavior of each species. To determine the definitive doses, first serial dilutions were made, however, not all were applied to the sample. A selection of five dilutions was made, based on assumptions of the mortality that they could cause, and after the evaluation, along with the analysis of the data, the tests were adjusted, adding or not adding other doses. Thus, the number of doses of each test was variable.
Determination of LD50 by means of topical application:
Topical application was performed using the Burkard® precision microapplicator. Each dose was applied to an experimental group of 30 bees, 10 bees from each hive, using three replicates (EPA, 2012b), plus an experimental control group without application, only was applied the aqueous solution with detergent and hold under the same conditions (Medrzycki et al., 2013; OECD/OCDE, 1998).
Each bee received 1 μL of the solution corresponding to each treatment on the pronotum (Medrzycki et al., 2013, OECD/OCDE, 1998, OEPP/EPPO, 2010); then the individuals were placed in a Petri dish to allow evaporation of the compound and subsequently deposited per replicate in an aluminum box and taken to the dark room where mortality and poisoning symptoms were observed at 4, 24 and 48 h (Medrzycki et al., 2013; OECD/OCDE, 1998).
Determination of oral LC50: Subjects were anesthetized to be placed in the aluminum boxes, where they were fed 10μL/bee of a 50% sucrose solution contaminated with the doses proposed for the test for 4 h (Medrzycki et al., 2013; OECD/OCDE, 1998; OEPP/EPPO, 2010); afterwards the contaminated food was removed and a tube with clean food solution was placed.
The number of boxes or experimental units was initially 18 for each test; but with increasing doses in some of them this number varied; however, all boxes were maintained under the conditions described above and observations were made with the same periodicity as in the previous evaluation. No repetition in time was performed.
Statistical Analysis: Due to the nature of the data and the objective of this work to determine the LD50 applied both topically and orally with their respective 95% confidence intervals and Chi square values, the Probit analysis was performed using EXCEL software, XLSTAT 2015 statistical package and GraphPad Prism software.
Results and discussion
Oral and topical lethal dose: Oral LC50 is a measurement that allows to obtain the lethal concentration of a pesticide in a food solution. However, its effect depends on the amount ingested by the target organism; LC50 is then converted into LD50, and represents the estimated amount of pesticide that causes the mortality of the organism. The oral exposure diet to reach the LC50 to thiamethoxam in T. angustula of 6.664 ng μL4 diet, and to S. xanthotricha of 0.1848 ng μL-1 diet was estimated. With fipronil the following data were obtained: T. angustula: 0.1864 ng μL4 diet and S. xanthotricha: 0.8162 ng μL1 diet.
The oral LD50 was calculated after considering the concentration of the pesticide in the solution with which the bees were fed, it was also necessary to estimate the average consumption of the specimens during the 4 h of exposure, in order to know the amount of pesticide they consumed and subsequently calculate the LD50 in ng/ bee; for S. xanthotricha it was 2 μL and for T. angustula 0.8 μL; results are presented, with the respective statistics, in Tables 2 and 3.
TABLE 2 Effect of thiamethoxam and fipronil o≠n the stingless bee Tetragonisca angustula.
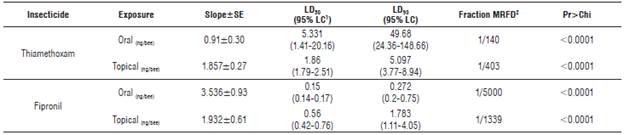
1 Confidencial level; 2 maximum recommended field dose.
TABLE 3 Effect of thiamethoxam and fipronil on the stingless bee Scaptotrigona xanthotricha.
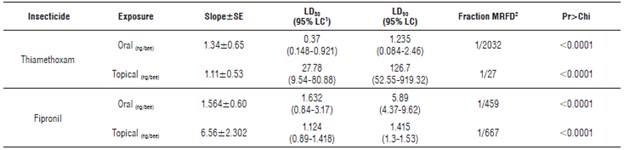
1 Confidencial level; 2 maximum recommended field dose.
Slope interpretation of the curve Dose-Response: Figures 1 and 2 show the model used to determine of the topical and oral LD50 of the two insecticides. The different doses corresponding to the estimated percentages of mortality can be identified in the graphs. In addition, the risk represented by the insecticides to the bees can be interfered from the data in Tables 2 and 3. The slopes resulting from the oral fipronil tests in T. angustula and fipronil topical in S. xanthotricha were superior to 2, indicating a high sensitivity of both species to small modifications in the used dose; therefore, the existing range between LD50 and LD90 in these two cases is less than in the other tests.
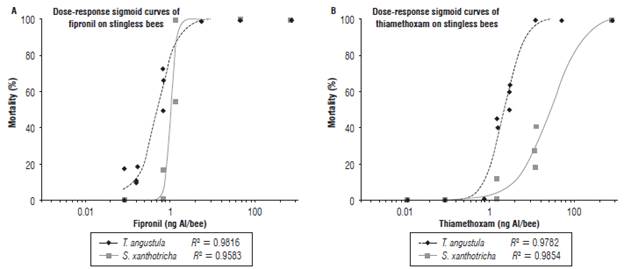
FIGURE 1 Dose curve response evidencing the lethal effects of a dilution series based on the respective MDRC of fipronil (A) and thiamethoxam (B) (1/1, 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128, 1/256, 1/512, 1/1024, 1/2048, 1/4096); topical application on T. angustula and S. xanthotricha (mortality was measured 48 h after treatment).
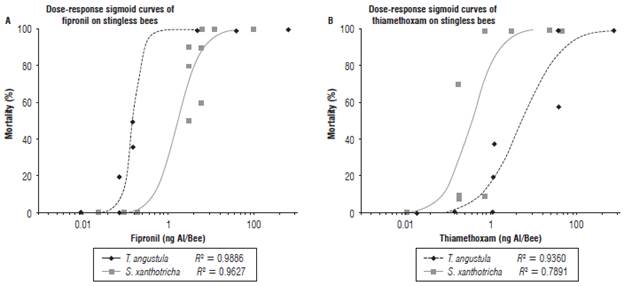
FIGURE 2 Dose curve response evidencing the lethal effects of a dilution series based on the respective MDRC of fipronil (A) and thiamethoxam (B) (1/1, 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128, 1/256, 1/512, 1/1024, 1/2048, 1/4096), oral exposure of T. angustula and S. xanthotricha. (Mortality was measured 48 h after treatment).
In the oral thiamethoxam test for T. angustula, the lowest slope inclination was obtained, which explains the great difference between the LD50andLD90; second is the thiamethoxam topical test on S. xanthotricha where the same behavior is observed between the two lethal doses. This low slope inclination agrees with the higher doses of the LD50 calculated in this study and make the different reaction of the two species to thiamethoxam evident. According to Zhu et al. (2015) the risk is influenced by the products concentration at field application and the slope of the dose response curve, a high inclination of a slope indicates a high sensitivity to the pesticide; small dose increase causes high mortality.
It is of great importance to know the sensitivity and the reaction of species to insecticides, based on the slope of the dose response curve. This slope describes the doses that may or may not cause a sub-lethal effect can be inferred and it also implies a contribution when planning the doses for toxicological studies of sub-lethal effects.
Routes of exposure to pesticides: There are multiple routes of exposure to pesticides that affect bees, being direct contact with a pesticide solution and with the powder from treated seeds or the application of pesticides in granular form to the soil the most common ones. Oral exposure to residues; or products translocated by the plant that are present in the pollen or nectar of planted or associated plants within or at the crops border and nearby crops; all the exposed aerial applications; the application of granules to the soil; the seed treatment or the absorption of residues from previous grown crops (EFSA European Food Safety Authority, 2013c; Godfray et al.,2014) are considered highly hazardous .In addition, it is assumed, that its response also depends on intrinsic factors, such as ecology, biology, physiology, morphology and extrinsic factors such as climate and flora.
No research was found on the consumption of free environmental water by stingless bees; however, a topical or oral exposure to insecticides can be potentially considered either by the guttation drops from some crops treated with systemics, the consumption of contaminated water from puddles or ditches or droplets present on plant surfaces or on soil as a remain product of the irrigation, as mentioned by Girolami et al. (2009) for A. mellifera. These studies should be carried out for stingless bees. It is of special importance to consider the pesticide or its metabolites risks and how to measure their effects, matter slightly studied so far, once these routes of exposure have been verified.
If bees use guttation drops to support the hive, it is important to study the content of thiamethoxam and its metabolites in plants such as Fragaria x ananassa (Strawberry), where the product is used by means of foliar application and in regular irrigation water. Strawberry flowers are those normally visited by T. angustula (Imperatriz-Fonseca et al., 2015). Many Gramineae such as Zea mays although they do not require bee-assisted pollination, could provide guttation droplets to bees after through secretions by maize and pollen. This solution has been found to contain thiamethoxam residues when treated seeds are planted or granules are applied to the soil (Godfray et al., 2014; EFSA European Food Safety Authority, 2013a, 2013b; Simon et al., 2013).
Intoxication symptoms: After topical application and oral exposure, acute poisoning symptoms were observed with the highest doses of both insecticides. Intoxication with fipronil of S. xanthotricha, initially induced a high excitation condition, represented by wing movement, vibration and circular movements at the same site. Later, slowness in movement, tremor and a displacement difficulty was observed, which also occurred in another stingless bee, M. scutellaris when exposed orally and topically to the same compound (Lourenço et al., 2012); T. angustula symptoms were less evident due to their slower movement dynamic, but it was also observed that individuals struggled with difficulty and trembled to death. After less than 4 h after the application event the individuals were dead showing a full spread of wings and legs. With thiamethoxam poisoning, individuals of both species started to move rapidly throughout the box; minutes later they slowed down and were paralyzed, agreeing with the symptoms observed for S. postica when exposed to imidacloprid (Soares et al., 2015); in some cases the bees died clinging to the mesh of the experiment boxes (Fig. 3). The dead individuals showed a swollen abdomen (Fig. 4) and legs and wings contracted.
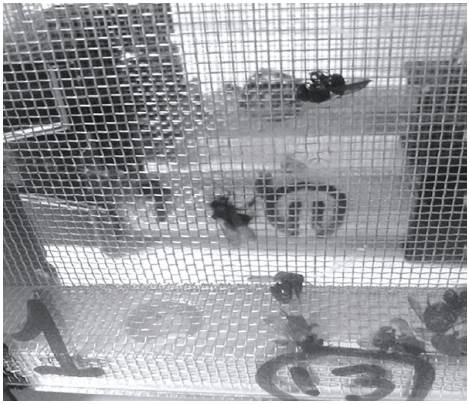
FIGURE 3 Scaptotrigona xanthotricha dead and firmly entangled on the mesh of the cage after topical exposure to thiamethoxam.
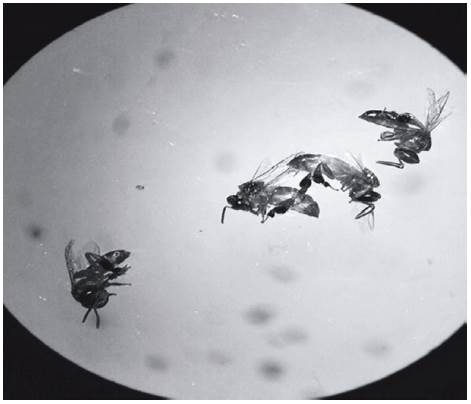
FIGURE 4 Tetragonisca angustula dead with swollen abdomen after oral contamination with thiamethoxam.
Toxicological comparisons: Fipronil has been evaluated on several species of stingless bees, as well as on domestic bee A. mellifera.Jacob et al. (2013) reported a topical LD50: 0.54 ng/bee and oral LC50: 0.24 ng μL-1 diet to S. postica. On the other hand, Lourenço et al. (2012) recorded a topical LD50 of 0.41 ng/bee and an oral LC50 of 0,011 ng μL-1 diet on Melipona scutellaris. Comparing the results of this study with those obtained by the mentioned authors, it is observed that both species are more susceptible than S. xanthotricha with both topical exposure and oral administration, whereas S. postica is slightly more tolerant to the insecticide than T. angustula provided in oral form, but in turn more tolerant than M. scutellaris. When A. mellifera was exposed topically to Fipronil, Vidau et al. (2011) obtained an LD50 of 417 ng/bee, which shows that both species are more susceptible than the domestic bee.In relation to thiamethoxam, Valdovinos-Nuñez et al. (2009) obtained a topical LD50 of 4 ng/bee to Nanotrigona perilampoides, which reflects a susceptibility, approximately 6 times greater than S. xanthotricha, but a higher tolerance to the product than T. angustula. Even so, the topical LD50 reported by Zhu et al. (2015) on A. mellifera was equivalent to 40 ng/bee, twice the obtained value to S. xanthotricha and more than 30 times greater than the LD50 for T. angustula.
Finally, the research carried out by Tomé et al. (2015) showed that Imidacloprid is lethal in doses used in the field for the S. xanthotricha, as has also been observed in the present study, where thiamethoxam and fipronil were lethal to all individuals of the two species, when exposing the bees to the doses used during field application.
The EFSA European Food Safety Authority (2013c) states that both bumblebees and solitary bees, because of their own biology, ecology and physiology, are more susceptible to pesticides than A. mellifera. Based on the results of this study, the stingless bees could be included in this concept amplifying EFSA's statement.
Comparing the susceptibility of both bees to insecticides, as well as their capacity to adapt to agroecosystems, allows to recognize their potential as pollinators that could replace Apis mellifera. Jaffe et al. (2015) found that Trigona snipes is able to colonize degraded environments and may persist in highly altered landscapes, being an example of a "rescue pollinator".
Susceptibility and risk: In general, a greater susceptibility of both species to fipronil was detected. S. xanthotricha also presents a high susceptibility to thiamethoxam in oral exposure. Furthermore, fipronil is toxic to the 50 and 90% of the sample in doses lower than 2 ng/bee. Representing a high toxicity after applying the classification criteria proposed by Atkins et al. (1981), which indicates that insecticides with LD50 less than 2 μg/bee (2,000 ng/bee) are highly toxic to this group of insects.
In the case of thiamethoxam, S. xanthotricha showed a higher tolerance to topical exposure than T. angustula, although there is a high toxicity to oral exposure. This result is considered concerning, due to the high systemic-ity of the product, which, being soluble in water, is rapidly translocated to all parts of the plant and represents a risk due to in foliar application, soil or seed treatment. Based on this mode of action, the European Union banned its use in many crops, considered attractive to bees or in those in which the bees could be exposed to the compound (The European Commission, 2013).
The maximum recommended dose for field applications represents the dosage suggested by the formulator of the commercial product. In both cases the dose from which the dilutions were made was 3 g L-1. Therefore, direct contact in the field during spraying or exposure to fresh or dry residues could be of great detriment to the survival of the hive of both species.
The evaluation of the potential risk of a pesticide requires not only the calculation of the LD50 but also the evaluation of larval toxicity, the effects on their behavior, the effect on the hypopharyngeal gland as well as the cumulative effect of the pesticides (EFSA European Food Safety Authority, 2013c). So far, no research on this topic for Meloponini has been carried out.
This basic research demonstrated the susceptibility of the T. angustula and S. xanthotricha to the action of thiamethoxam and fipronil when administered orally or topically. A greater susceptibility of these two species of native bees than that of A. mellifera to these pesticides was confirmed. There are several reasons that could explain this greater susceptibility, such as an adaptation and selection processes of Apis being over time exposed to insecticides, size and the presence or absence of structures that could protect from direct contact with the compound, foraging habits associated with weather and the moment an application is made, among other causes.
There is very little information and studies on stingless bees and without them it is impossible to establish with any certainty the actual risk represented by pesticides for these bees. It is of great importance to carry out studies on native stingless bees, taking into account their role as pollinators in the diversity of fauna and flora. At present for registration and use of pesticides only the LD50 on A. mellifera is considered, without knowing fully the real risk even on this species. The consumption of pollen and nectar has to be quantified, as well as the use of guttation water, to evaluate all routes of exposure and to fully identify the risk that for the meliponines can represent pesticides, used under field conditions.
In front of CCD and the continuing degradation of ecosystems, meliponines can play a crucial role regarding global food production and food security, up to now, no reports of CCD affecting meliponine hives have been reported, probably due to differential characteristics of A. mellifera and stingless bees. Eventually they could recover the role they had as pollinators before the introduction of the A. mellifera in the tropical and subtropical region.
The great Meloponini biodiversity richness of Latin America is, among many others, a justification to increase efforts in native bee species conservation and reducing the impact of agriculture practices on ecosystems.