Introduction
Foodborne diseases are the most serious and expensive issues in food industry. According to the USDA (United States Department of Agriculture) annually in USA, 48 million people suffer foodborne illnesses and 3000 are reported as deadly cases (FDA and Administration, 2013). New meals, manufacturing processes, and the growing demand for minimally processed products (ready-to-eat) increase the possibility of microbiological contamination. Alternative food preservation technology such as bio-preservation is a reliable option to extent the shelf-life and to enhance the hygienic quality, minimizing the impact on the food nutritional and organoleptic properties (García et al., 2010). Bio-preservation uses the antimicrobial potential of non-pathogen microorganisms or their metabolites to inhibit the growth of pathogens or spoilage related microorganisms (Nath et al., 2014; Settanni and Corsetti, 2008; Smid and Lacroix, 2013).
Different strains of microorganisms with potential use as bio-preservative agents have been reported (Ghanbari et al., 2013; Henning et al., 2015; Hwanhlem et al., 2014). Dairy products are one group of foods commonly used to obtain strains with antagonistic features. The most important microorganisms with antagonistic characteristics and potential use in food industry are lactic acid bacteria (LAB). They have been traditionally associated to food and are considered safe (García et al., 2010). LAB are Gram positive bacteria, non sporulating, anaerobic facultative, catalase and coagulase negative, tolerant to acidic conditions and with low content of guanine and cytosine in their DNA. LAB belong to the group of Firmicutes, Lactobacillales order and the most representative genera are Aerococcus, Alloiococcus, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Symbiobacterium, Tetragenococcus, Vagococcus, and Weissella and in phylum Actinobacteria with genera Atopobium and Bifidobacterium (Giraffa, 2012). Many LAB are considered as probiotics or live microorganisms, that consumed in adequate amounts, confer a health benefit on the host (Fijan, 2014).
LAB are successful habitat competitors due to their ability as competitive exclusion (Settanni and Corsetti, 2008) which consists on releasing antimicrobial substances that are able to affect the development of other microorganisms (Smid and Lacroix, 2013). The main product of LAB metabolism is lactic acid but they can produce other organic acids and compounds as hidrogenus peroxide (H2O2), carbon dioxide (CO2), diacetyl (2,3-butanediona), reuterin and bacteriocins (Ammor et al., 2006; Khan et al., 2010; Nath et al., 2014). Organic acids, especially lactic acid, are metabolites produced as a result of sugar metabolism. They are released to the environment reducing its pH, inhibiting the development of some populations of undesirable microorganisms (Okano et al., 2010). Hydrogen peroxide (H2O2) has an oxidizing effect over sulfhydryl groups of membrane proteins and over lipids, also damaging the cell wall of some other microorganisms (Finnegan et al., 2010). Additionally, H2O2 reacts with O2, forming CO2 reducing free O2 and creating an anaerobic environment that can reduce the development of anaerobic populations (Suskovic et al., 2010). Diacetyl is associated to a characteristic smell of some dairy products, but it also has antagonistic activity when interacts with the cell membrane of some bacteria altering some metabolic ways (Lanciotti et al., 2003). Bacteriocins are antimicrobial peptides produced by Gram positive and negative bacteria. In general, those peptides have low molecular weight and are heat stables, they do not affect the producer cells due immunity mechanisms (Cotter et al., 2005; Karumathil et al., 2016).
In this work, were isolated and identified LAB with antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes, three of the most important foodborne pathogens. These results are very important for the possibility of their application on bio-preservation of foods.
Experimental procedures
Isolation and identification
Lactic acid bacteria were isolated from raw milk obtained from the Veterinarian Medicine Faculty of the Universidad Nacional de Colombia. Briefly, milk was incubated at 37°C during 48 h in order to allow fermentation and to obtain bacteria strains that survive on acid milk. 11 ml of fermented milk were added to 99 ml of sterile peptone water (0.1%) and tenfold dilutions until 10-7 were made. 1 ml of dilutions 10-5, 10-6 and 10-7 were poured in petri dishes and covering with MRS agar (Man, Rogosa and Sharpe) Oxoid, petri dishes were incubated at 37oC during 48 h. In order to obtain more diversity in the isolation, colonies with different morphologies were plated onto the surface of MRS agar. After incubation at 37oC during 24 h, Gram stain was performed. Isolates were stored at -20oC on cryovials (CRIOBANK®).
In order to know some features of the isolates some biochemistry tests were performed. Gas production test in Durham tubes on MRS broth; growth in MRS broth at different temperatures (10, 30, 37 and 45oC) and tolerance to NaCl 6.5% tests were performed. Growth was measure as optic density (O.D) on spectrophotometer (Genesys, USA) at 600 nm. Catalase and oxidase were also tested (Muñoz et al., 2012). Isolates were partially identified on the basis of their biochemical features according to fermentation ability by using API 50CHL (Api System S.A., Bio-Merieux, France) (Todorov et al., 2013).
To obtain the molecular identification, isolates were activated in 5 mL of MRS broth (Oxoid, UK) and incubated at 37oC during 48 h. After incubation the DNA was extracted using the kit PureLink™ Genomic DNA Mini Kit (Invitrogen) following manufacture instructions. Extraction was verified by electrophoresis in agarose gel 1.4% (w/v) at 70 Volts, 400mA during 30 minutes and the DNA was stained with SybrSafe. Concentration was measured by spectrophotometry using Nanodrop (Thermo Scientific, UK). Genera and specie were determined by amplification and sequencing of 16S ribosomal subunit by PCR technique using a Veriti® (Life Technologies, UK) thermo cycler. Conditions of PCR were: 94°C for 5 min, 30 times 94°C for 30 s, 55°C for 30 s, 72°C for 1.5 min and 72°C for 7 min. Reaction mixture contained 38.2 μL of water, 5 μL of buffer 1X, 1.5 μL MgCL2 1.5 Mm, 0.4 μL DNTs 0.2 Mm, 1.25 of primers (27F: 5' AGAGTTTGATCMTGGCTCAG 3', 1492R: 5' TACGGYTACCTTGTTACGACTT 3'), 0.4 μL Taq and 2 μL of DNA (Doi et al., 2013). Amplification products were separated by electrophoresis in 1.2% agarose gel. Amplified samples were sent to the Instituto de Genética (SSiGMol) in the Universidad Nacional de Colombia (Bogota). Samples were purified and sequenced in bidirectional way. The sequences were compared to those deposited in GenBank, using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST) and phylogenetic tree was constructed using the MEGA 7.0.14 software.
Antimicrobial activity screening
Screening to find isolates with antagonistic features was performed using the spot-on-lawn method described by Schillinger and Lucke (1989) with some modifications. Briefly, overnight cultures of the isolates were spotted onto the surface of agar plates with MRS (1.2% agar) and incubated for 24 h at 30°C to allow colonies to develop and produce their metabolites. Approximately 5 x 107CFU/mL of the indicator strains important in food industry (Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Listeria monocytogenes ATCC 7644) were inoculated into 100 ml of soft TSA (Trypticase soy) agar (containing 0.7% agar), and poured over the plate in which the isolated LAB were grown. After incubation at 37oC during 24 h, diameter of inhibition zones was measured from the edge of the zone with a caliper, and expressed in mm.
Results
64 lactic acid bacteria were isolated from raw cow milk. 23.1% of all isolates were Gram positive and 76.9% of them presented rod shape, minor proportion presented sphere shape. Rods were large and short, some of them thinner than others, they were arranged on pairs or short chains. Most of cocci were arranged on tetrads and some of them on pairs. 24 isolates were chosen for further analysis according to their antimicrobial activity potential.
The isolates growth at 6.5% of NaCl and at temperatures of 10, 30, 37 and 45°C (Fig. 1) showed that the optimal temperature for all isolates was 30oC followed by 37oC, some of them grew at 10oC and at 45oC was the least suitable temperature for growth. 46.13% of isolates were tolerant to 6.5% of NaCl. Those tests allow knowing the technological potential of isolates in food industry applications. Gas production was negative for all cases.
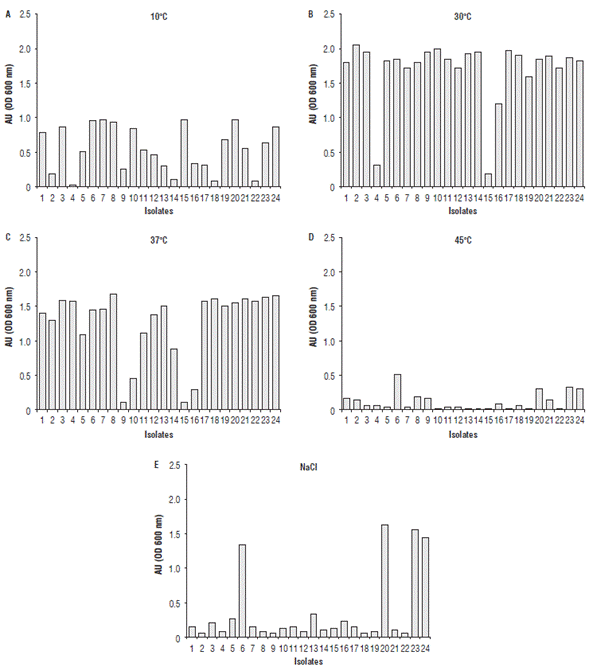
FIGURE 1 Growth of isolated LAB at different conditions of incubation,(A) 10°C, (B) 30°C, (C) 37°C, (D) 45°C, and (E) with 6.5% of NaCl. Numbers represent the isolated LAB; AU = absorbance units.
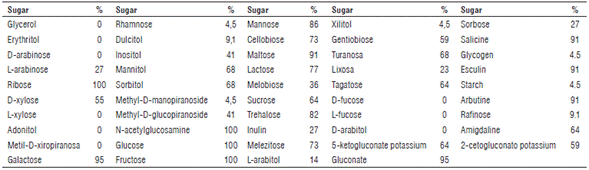
The isolates that presented rod morphology (19 of them) were tested with API 50CHL (Api System S.A., Bio-Merieux, France) designed as Lactobacillus sp. according with the manufacturer, in order to determine phenotypic characteristics showed in biochemistry profiles (fermentative profiles). Tab. 1 shows the percentage of isolates that were able to use each sugar on the API CHL 50 panels. All of the isolates fermented N-acetyl glucosamine, glucose, ribose and fructose and none of them was able to use D-fucose, L-fucose, D-arabitol, erythritol, D-arabinose, L-xylose, adonitol, methyl-D-xiropyranose or glycerol. Galactose and gluconate were used almost for all isolates, except for the identified at molecular level as Pediococcus, this result can be explained because some strains of this genera do not use the sugar sources mentioned (Vos et al., 2009). Arbutinine, aesculine, maltose and salicin were consumed by 89.5% of the isolates. Sugars consumed for one strain were rhamnose, metyl-D-manopiyanoside, starch, glycogen o xylitol. Partial identification according to biochemical profiles gave as a result that isolates belong to genera Lactobacillus spp. with species Lb. brevis 16%, Lb. plantarum 21%and Lb. paracasei spp. paracasei 63%.
Molecular identification
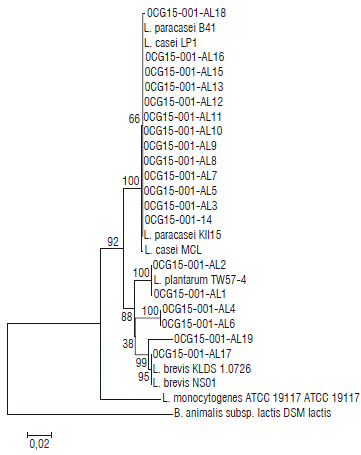
DNA extraction was performed to 19 isolates. Molecular weight marker of 1000 pb was used in order to know size of the product amplified. This size was approximated to 1400 pb according to observed bands. Chromax software was used to observe, interpreter, and depurate sequences obtained from the Genetics Institute (Universidad Nacional de Colombia, Bogota). Results were analyzed and compared to data in GenBank using BLAST (Basic Local Alignment Search Tool), phylogenetic tree was made using Mega 7.0.14 program (Fig. 2).
13 of the isolates were congregated on Lb. casei/paracasei cluster with a similarity percentage of 66% (isolates AL3, AL5, AL7, AL8, AL9, AL10, AL11, AL12, AL13, AL14, AL15, AL16 and AL18). Six isolates remaining were associated with 100% of similarity with Lb. plantarum (isolates AL1, AL2, AL4 and AL6), and Lb. brevis with 99% of similarity (isolates AL17 and AL19). According to the bioprospection concept about the obtaining of bioactive products from nature, in this case for applications in the food industry, the results showed the possibility to considerate the raw milk as an important source of antagonistic Lactobacillus strains with potential application on biopreservation. The above depends mainly of the natural behavior of bacteria due that the strains express different survivor strategies, as the release of antagonist substances as response to population density (Cornforth and Foster, 2013).
Antimicrobial activity evaluation
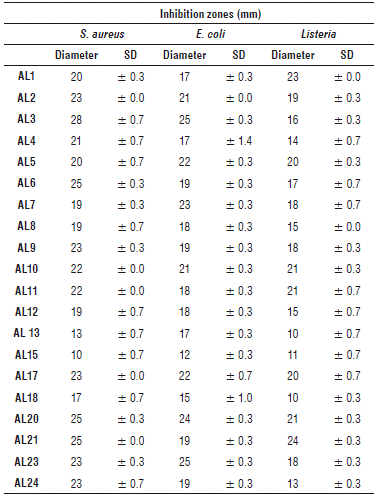
Fig. 3 shows the growth of the isolated BAL on MRS agar (1.2%) after incubation for 24 h at 30oC and the inhibition zones obtained after a second incubation with E. coli ATCC 25922, S. aureus ATCC 25923 and L. monocytogenes ATCC 7644.
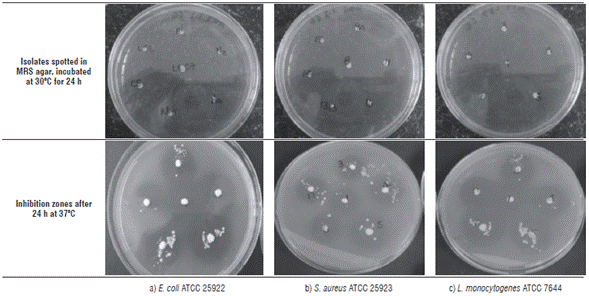
FIGURE 3 Antimicrobial activity screening. Upper part shows the growing of isolated LAB, the lower shows inhibition zones against E. coli ATCC 25922, S. aureus ATCC 25923 and L. monocytogenes ATCC 7644 performed by the isolated strains.
Tab. 2 shows inhibition zones in millimeters against indicator strains.
Discussion
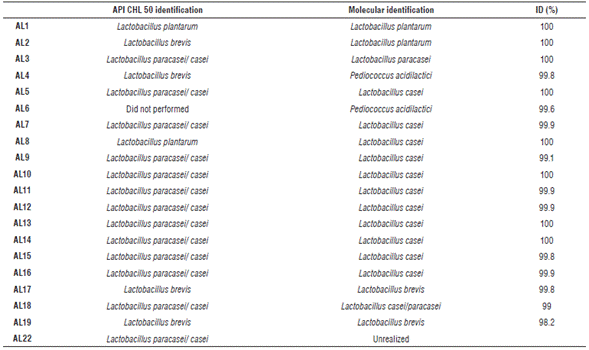
Biochemistry tests made by using API® CHL50 (BioMerieux, France) showed metabolic characteristics of the isolates, this information allowed to estimate sugar fermentation profile allowing growth media optimization. According to the results obtained on apiwebTM, 11 strains corresponded to Lb. paracasei, 9 of them were identified at molecular level as Lb. casei, one of them as Lb. casei/paracasei, and one as Lb. paracasei. The close related species were Lb. casei, Lb. paracasei, and Lb. casei/paracasei and they were all identified by the apiwebTM as Lb. paracasei. On the other hand, those profiles are not enough to identified microorganisms, so it is necessary to use molecular techniques to obtain more robust results. Tab. 3 shows that names obtained by API CHL 50® to isolates AL2, AL4 and AL8 did not correspond to the identification obtained by sequencing and contrasted with databases.
Two out of the 19 strains identified at molecular level corresponded to Pediococcus sp. The rest were verified as belonging to Lactobacillus sp. (Tab. 3). Five different species of lactobacillus were identified as Lb. casei, Lb. brevis, Lb. paracasei, Lb. casei/paracasei, Lb. plantarum. One species of Pediococcus was identified as P. acidilactici. Other researches focused on isolated of native LAB from different foods have shown that dairy products are an important source of this kind of microorganisms (dos Santos et al., 2014). For instance, 319 strains were isolated from different products made with raw buffalo milk on Gansu province (China), authors amplified and sequenced DNA from those isolates and compared results with GenBank database finding genera as Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, Enterococcus and Weissella. Major proportion of isolated corresponds to Lb. casei and Lb. helveticus (Bao et al., 2012). Sixty different isolates from five Spanish cheeses made without starter microorganisms were identified as Lactococcus lactis (Alegría et al., 2010). Davati et al. (2015) isolated 64 LAB from camel milk and using Amplified Ribosomal DNA Restriction Analysis (ARDRA) found 12 different profiles. In the same study, identification by amplification and sequencing of 16S region of DNAr were performed and P. pentosaceus, E. faecium cepa Y-2, E. faecium cepa JZ1-1, E. faecium cepa E6, E. durans, E. lactis, Lc. mesenteroides, Lb. casei and W. cibaria were found.
Other authors obtained and characterized morphologically isolates from paddy rice silage. API CHL50 tests to make the partial identification were performed. Also, analyses of 16S rRNA and recA sequences were made to obtain genera Lactobacillus, Lactococcus, Pediococcus, Enterococcus, and Leuconostoc. P. pentosaceus was the more abundant microorganism, and the low was Lb. casei (Ni et al., 2015).
Species most commonly isolated from raw milk belong to genera Enterococcus (E. faecalis and E. faecium), Lactobacillus (Lb. delbrueckii subsp. Lactis, Lb. helveticus, Lb. hilgardii. Lb. fermentum, Lb. gasseri and Lb. rhamnosus), Lactococcus (Lc. lactis subsp. lactis and subsp. Cremoris), Leuconostoc (Ln. mesenteroides subsp. mesenteroides), Pediococcus and Streptococcus (St. Uberis and St. thermophilus) (Neviani et al., 2013). Also, Lactobacillus sp. and Streptococcus sp. genera has been reported from raw cow milk (Elgadi et al., 2008). Other authors identified Enterococcus spp. and some members of Lactococcus spp., also they reported a less amount of Lactobacillus strains in contrast with the present work (Franciosi et al., 2009). Other authors isolated 7 species of Lb. plantarum from donkey milk. Results obtained on RAPD-PCR spectrum showed that isolates were replicates of the same strain, for this reason they evaluated one single strain named Lb. plantarum LP08AD. Bacteriocin LP08AD was identified and characterized at biochemistry and molecular level, also antimicrobial activity was measure against L. monocytogenes, E. faecium, Lb. curvatus, Lb. fermentum and P. acidilactici (Murua et al., 2013).
All strains presented antagonistic activity against L. mono-cytogenes, E. coli and S. aureus showing a potential application on research related with production and recovery of antimicrobial metabolites with broad spectrum against foodborne pathogens. Similar results have been reported with isolated strains belonging to genera Pediococcus, Enterococcus, Leuconostoc and Lactobacillus showed antimicrobial activity against S. aureus, Bacillus cereus and E. coli (Davati et al., 2015). In other study, 17 of the 60 of Lc. lactis isolated presented antimicrobial activity against Gram positive bacteria from the Lc. lactis cluster (different subspecies), Lb. sakei CECT 906T, Lb. plantarum LL 441, L. innocua 86/26 and S. aureus CECT 86T (Alegría et al., 2010).
Bioprospecting as a tool in the search for natural substances with potential applications in food is booming worldwide due to the need of developing food products with natural features that allow to replace the use of synthetic additives. This work showed the importance of raw milk as source of LAB with antagonistic against pathogen bacteria features and showed the needed to focus the research in the evaluation of the antimicrobial activity of the native LAB to characterize metabolites responsible of this activity with application in biopreservation of food.