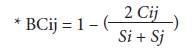Introduction
Cotton (Gossypium hirsutum L.) is an important crop highly related to the economic, social and agro-industrial development of many communities. in Colombia there are approximately 10,284 ha planted with cotton, mainly distributed in the Caribbean, Orinoquia and andean regions. The andean region represents 7,000 ha in the Magdalena warm valley which corresponds to the departments of Tolima, Huila and Cundinamarca. The national average yield is 1,971 kg of fiber ha-1 (Conalgodón, 2018).
The cotton industry generates 10,945 direct jobs with a production cost of $4.5 million pesos/ha (Conalgodón, 2018). As a strategy for pest management, the Instituto Colombiano Agropecuario (ICA) implemented the plan for the exclusion, suppression and eradication of cotton pests (ICA, 2000) in all producing regions. in this context, there are two annual planting seasons, the first one in the first half of the year in the Andean region, and the second one at the end of the second semester in the Orinoquia and Caribbean regions (ICA, 2000).
The Thripidae family comprises important pests of cotton because they are associated with direct damages such as leaf distortion, defoliation, bud and boll abortion, premature boll opening, and indirect damage such as viruses transmission that can affect yield and fiber quality (Wilson and Bauer, 1993; Leigh, 1995; Cermeli et al, 2009; EFSA, 2014; ThripsWiki, 2017). In the global context of cotton production, the genera Caliothrips Daniel, Retithrips Marchal, Sericothrips Haliday, Thrips Linneaeus, Frankliniella Karny and Scirtothrips Shull have been registered (Wilson and Bauer, 1993; Leigh, 1995; Mailhot et al., 2007; Kumar et al., 2013).
The insect S. dorsalis Hood, 1919 is a polyphagous species of tropical Asian origin (Ananthakrishnan, 1993) that feeds on young leaves of more than 200 species of dicotyledonous plants in about 40 different botanical families (Hoodle and Mound, 2003; Hooddle et al., 2008). S. dorsalis feeds preferentially from the epidermis and, sometimes, from palisade tissues and tissues of the apex of young fruits, especially when they are still hidden under the calyx. In many hosts, it can feed on the upper surface when infestation levels are high. Larvae and adults are often located in the midrib or near the damaged leaf tissue area. Pupae can be found in leaf litter, leaf axils, and deformed leaves or under the calyx (Kumar et al., 2013). The associated damage in plants infested by S. dorsalis is characterized by a silvering on the surface of the infested leaf, feeding scars, linear thickening of the leaf blade, and brown spots on leaves and fruits. A concentric ring often appears in healed tissue around the apex. Poor fruit formation and early leaf senescence take place as well (Hodges et al., 2005; Kumar et al, 2013).
Thrips S. dorsalis are small insects (<1 mm in length), with thigmotactic behavior and similar morphology features as other thrips species (Kumar et al., 2011). It is a species of great economic importance in the world, which was registered for the first time in Colombia in 2010 (ICA, 2012). Due to this, it is necessary to evaluate the potential and actual phytosanitary impact of the species at regional and national levels. The objective of the present study was to determine the infestation and preferences of S. dorsalis for the structures of young leafs, floral buds, flowers and bolls (fruits) in cotton plants from the Magdalena warm valley of Colombia.
Materials and methods
Study area
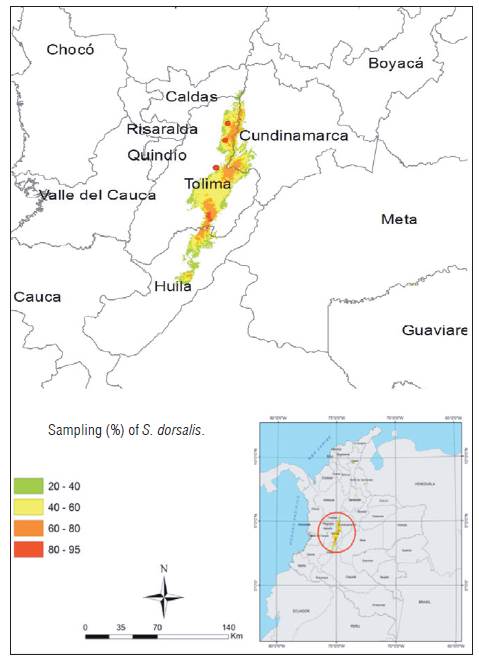
The geographical context of this study corresponded to the Andean region, the Magdalena warm valley in the departments of Huila, Tolima and Cundinamarca. The research area presents an altitudinal range below 600 m a.s.l., characterized by tropical dry forest vegetation (bs-T) (Holdridge, 1967; IAvH, 2014). Farms were inspected in the municipalities of Villavieja, Campoalegre (Huila); Venadillo, Natagaima, Ambalema, Lerida, Armero, Espinal (Tolima) and Ricaurte (Cundinamarca) (Fig. 1).
Sampling
The sampling included 60 farms with productive cotton crops (60 to 90 d after sowing) with floral buds, opened flowers and bolls (fruits). All cotton crops corresponded to genetically modified or transgenic varieties. Ten cotton plants were randomly selected per hectare in each plot. Five young leaves or leaf terminals, five floral buds, five opened flowers and five bolls or fruits were randomly inspected in the lower, middle or upper strata of each plant. The thrips were collected using brushes previously moistened with ethanol and deposited in 1.5 mL plastic vials with 70% ethanol. The sampling unit was represented by 20 vials per inspected plant. In each plot, a code that represented the geographical location, the number assigned to the plant and the plant structures B (buds), C (boll), F (flower), H (leaf), P (farms) were assigned (Pedigo, 1996). The samples were packed in plastic bags according to the farm and municipality, and were transported to the Laboratories of Entomology of the Nataima research center of the Corporación Colombiana de investigación Agropecuaria AGROSAVIA (Espinal-Tolima) and BIOQUALITYAGRO in the municipality of Mosquera, Cundinamarca, with the purpose of preparing the specimens and carrying out the taxonomic identification. The geographical coordinates for some localities were obtained and/or corrected using Google Earth (www.googleearth.com) and the map was made in Arcmap (ArcGis) software (Pulido et al., 2015).
Sample treatment
Immature stages were separated from the adults and a first classification was made according to the present thrips morphotypes, separating the adults of possible S. dorsalis specimens from the others. The number of adults was recorded and the specimens were rinsed using 5-10% KOH.
Then the specimens were washed with distilled water and dehydrated in ethanol for semi-permanent mounting in Hoyer's solution on slides (Mound and Marullo, 1996). The taxonomic identification was made based on the morphological characters and according to the available keys (Hoddle and Mound, 2003; Moritz et al, 2007; Kumar et al., 2013), and with the aid of a Leica* ZOOM 2000 stereoscope (Wetzlar, Germany) and a Nikon* Type-119YS2-T microscope (Tokyo, Japan) and photographic camera Canon* Type SX530-16MP (Tokyo, Japan).
Data analysis
The abundance of S. dorsalis adults in each plant structure (young leaf, floral buds, opened flower and boll) per plant, per plot, in each municipality and department evaluated was determined. Considering this information, the percentages of infestation per plot and the preference of S. dorsalis for certain plant structures in cotton plants were estimated. T-Student and Kruskal-Wallis tests were performed in order to find statistical differences between the different evaluated variables (Ebratt et al., 2004). The selectivity of S. dorsalis for each plant structure was determined by Z tests (P<0.05) with paired comparisons per structure, and Spearman correlation analysis between infested plant structures (STATISTICA v10; infoStat, 2016). Percentage records of the calculations to obtain the Bray-Curtis similarity index* were transformed on a logarithmic scale in base 10, in order to define the preference for the resource as follows: a) positive preference (I>1), b) neutral or accidental preference (I = 1), and c) non-preference (I<1). This is a statistic used to quantify the compositional dissimilarity between two different sites, based on counts at each site:
where C ij Cij is the sum of the lesser values for only those species in common between both sites, while S i and S j Si and Sj are the total number of specimens counted at both sites (Silveira-Neto et al., 1976; Johnson, 1980; Ramirez, 2006; Zamar, 2011; Yara and Reinoso, 2012).
Results
Presence and distribution
A total of 12,000 plant structure samples were obtained. These samples corresponded to 30 farms in the department of Tolima (10 in the northern zone, 10 in the central zone and 10 in the southern zone), 20 farms in the department of Huila (10 in the northern zone and 10 in the central zone) and 10 farms in the southwestern zone of Cundinamarca.
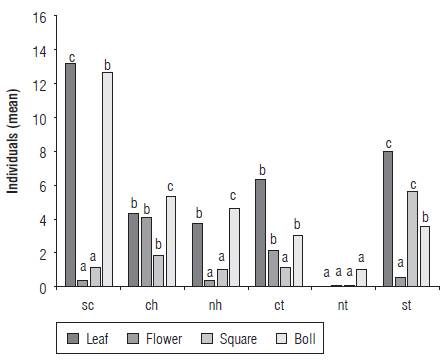
The thrips S. dorsalis was found in 46 farms (77%) with infestation rates between 20 and 100% (Tab. 1). The species was not recorded in 14 farms (23%) located in the municipalities of Armero (5 farms), Ambalema (1 farm), Venadillo (1 farm), El Espinal (5 farms), Natagaima (2 farms), all located in the department of Tolima. The infestation in bolls reached 61.6% (n = 60 ± 6.33), followed by young leaves with 55% (n = 60 ± 6.47), floral buds with 41.66% (n = 60 ± 6.41) and opened flowers with 28.33% (n = 60 ± 5.86). In all cases, S. dorsalis was found to be associated with young leaves and bolls (n = 60; R = 0.4592; P<0.001) whereas flowers and floral buds showed low abundance (C = 0.85; gl = 3; H = 19.65; P<0.0001) (Fig. 2). The south of Cundinamarca and south of Tolima presented the highest abundances in young leaves, bolls and floral buds. The same preference was found in the central and northern zones of Huila and in the central area of Tolima, but with significantly low abundances. In the northern region of Tolima, S. dorsalis abundances were significantly lower in young leaves, flowers, floral buds and bolls (C = 0.94; gl = 5; H = 19.89; P<0.0008) (Fig. 3).
TABLE 1 Sampling and abundance location of S. dorsalis Hood (Thysanoptera: Thripidae) in cotton crops from the Magdalena warm valley, Colombia.

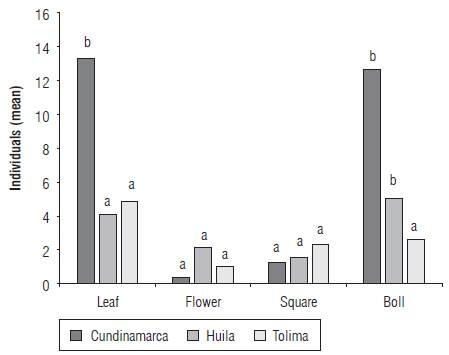
FIGURE 2 Abundance of S. dorsalis on plant structures of the cotton crop per department (C = 0.85; gl = 3; H = 19.65; P<0.0001).
Preference for structures
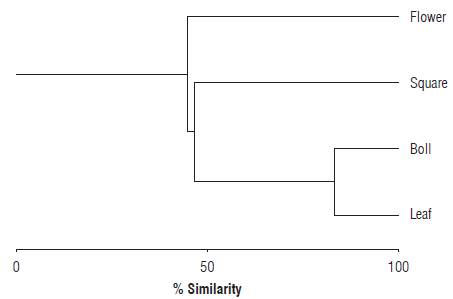
According to the similarity indices (is) applied, significant differences (P<0.05) were found in the abundances of S. dorsalis between structures in the cotton plant: leaf-flower (is = 0.40), leaf-bud (is = 0.71), leaf-boll (is = 0.52), flower-bud (is = 0.24), flower-boll (is = 0.16) and bud-boll (is =0.78). The Bray-Curtis similarity-affinity index showed significant differences between the paired structures flower-boll (Rs = 0.6742; P = 0.01), flower-leaf (Rs = 0.5900; P = 0.006) and boll-leaf (Rs = 0.5277; P = 0.017). However, no significant differences were observed between the paired structures flower-bud (Rs = 0.4244; P = 0.062) and boll-bud (Rs = 0.4037; P = 0.077). The preference analysis based on the abundance of S. dorsalis in the evaluated structures revealed that the species had a higher affinity for the bolls (I = 1.7>1), followed by young leaves (I = 1.3>1) and buds (I = 1.2>1). Opened flowers were not a frequented resource for the pest with a similarity range of I = 0.8 (<1) (N = 60; cophenetic correlation = 0.927; read cases = 60; omitted cases = 0; P<0.05). Paired comparisons using the Z test (P = 0.05) revealed that there were significant differences between boll-flower and young leaf-flower (Fig. 4).
Discussion
This study indicates that S. dorsalis was found to be associated with cotton crop plants of the Magdalena warm valley in Colombia, with a preference for young leaves, floral buds and bolls. It is clear that these microhabitats have sufficient food and reproductive resources to complete the life cycle and maintain the populations of the species (Mound and Stiller, 2011), which poses a high risk for crop yield and profitability (Mound and Stiller, 2011; Mannion et al., 2013). it has been reported that leaves and other non-flowering structures may be more stable food sources for the development of immature stages (Funderburk et al., 2002), contrasting the information described to the genus Frankliniella Karny, strongly associated with flowers due to the fact that pollen constitutes an essential nutritional contribution to increase egg production. in general, it is understood that young leaves have a higher nutritional content, but also more secondary metabolites, so insect populations must develop efficient strategies to regulate their intake (Schoonhoven et al., 2005), as observed in S. dorsalis, which prefers to feed around the main veins of the leaves or in areas bordering damaged zones (EFSA, 2014).
Due to the fact that immature stages of S. dorsalis were found during the sampling performed in the present study, it is suggested that cotton plants constitute true hosts where the species can develop and complete its life cycle (Mound and Marullo, 1996; Marullo, 2004; Marullo, 2009; Vierbergen et al., 2010; Alves-Silva et al, 2013). This result is relevant because there is scarce knowledge about host plants and feeding strategies of the genus Scirtothrips, which has been a barrier for the definition of surveillance and control strategies (Zwölfer, 1983; Lacasa et al., 1996; Mound and Marullo, 1996; Hall et al., 1997; Pérez, 1999; Hernandez, 2005; Alves-Silva, 2013; Kumar et al., 2013; Burckhardt et al., 2014). The specialization in insects corresponds to the general rule and not to the exception (Pérez, 1999; Schoonhoven et al., 2005), so that the recognition of preferences could offer important ethological elements in the differentiation of the species and could also give clarity on their economic importance (Thorsteinson, 1960; Stern, 1973; Zwölfer, 1983; Bernays and Chapman, 1994; Sha et al., 1998; Zamar and Neder, 2012; Cook et al., 2013). In the present study, symptoms such as leaf distortion (leaf curl), leaf wilting, leaf fall, and discoloration of boll and floral buds (bronzing), were attributed to the insect presence in the cotton plants (Fig. 5).
The insect S. dorsalis is an introduced species in Colombia, with a type "r" reproductive strategy and records of polyphagia (Morse and Hoddle, 2006; Rodríguez, 2006; Lieb-hold and Tobin, 2008). All the above, in conjunction with the distribution recorded in the present study, can generate negative effects on biodiversity, uncultivated native flora and other crops of economic interest such as mango, citrus, avocado, tomato, chili and aromatic herbs, which are also established in the Magdalena warm valley in the Andean region of Colombia (Mound and Palmer, 1981; Hoodle and Mound, 2003; Liebhold and Tobin, 2008; Kumar et al., 2011; Kumar et al., 2013; Wegier and Piñero, 2013). It is clear that the introduction and establishment of non-native species affect trophic networks and can alter processes of population dynamics that allow species regulation (Lewis, 1997; Schoonhoven et al., 2005; Gutiérrez, 2006). This fact must be considered as an important risk if the cultivated area is a cotton monoculture.
Considering the percentages of infestation and insect preferences found for cotton crop in the Andean region of Colombia, it is clear that S. dorsalis could become a major phytosanitary problem that should be carefully monitored because: a) it could adversely affect the expected yields and the quality of the fiber, b) it produces direct damages that cause the fall of leaves, floral buds and bolls, c) it favors the distortion in leaf blades in the shape of rosettes, d) it contributes to the foliar malformation, e) it can generate dwarfism and death of the plant (Fig. 6). Several researches have established that S. dorsalis causes indirect damage related to the transmission of viral particles such as Groundnut chlorotic fan-spot virus, Groundnut yellow spot virus and Tobacco streak virus, as recorded in cotton crops in Australia and the United States of America with a reduction of 77% in seed production, 25% in yield and 67% in fiber quality, (Funderburk, 2002; Jones, 2005; Shukla et al., 2005; Riley et al, 2011; EFSA, 2014). it has been found that Frankliniella tritici may be associated with cotton flowers (10%) causing detriments due to its feeding, but also as a carrier of the fungus Fusarium verticillioides, causal agent of the so-called Hard Lock syndrome (Mailhot et al., 2007), which coupled with the damage caused by S. dorsalis to cotton plants, constitute important risks for the cotton production chain. However, it is interesting that the insect was not present in 14 of the total farms evaluated (23%), which could be explained by agronomic management practices based on the regular use of insecticidal molecules of chemical synthesis, or due to abiotic factors that do not favor the establishment of the species. The entomological surveillance of the species should be continued, not only in cotton crops but in other plants that can serve as hosts, which can be established through models of distribution and ecological niche of the species (Pulido et al, 2015).
Conclusions
The importance of the association of S. dorsalis in cultivated cotton plants described in the present study leads to opportunities to perform projects oriented towards biology, ethology and the recognition of natural enemies of this pest species. Those projects may allow the definition of strategies of integrated pest management and the evaluation of the impact on the cotton production chain for the country.
The tropical dry forests correspond to a life zone where agriculture has grown with many extensive crops such as chili, mango, rice and cotton, which have been reported as host plants for S. dorsalis. This species has been dispersed and established in this region because it is considered as a favorable ecological niche in this area.
The introduction and establishment of species such as S. dorsalis could affect the trophic networks present, altering processes of population dynamics that allow the regulation of the species. This, for the present study, would constitute an important risk to the evaluated areas, if these areas have appropriate environmental conditions as ecological niches for the establishment of the invasive species.