Introduction
The production of exotic fruits in Colombia has been increasing in recent times due to the tropical conditions that allow a constant production and a permanent fruit supply over the year (Contreras et al., 2011). The andean blueberry Vaccinium méridionale Swartz (Ericaceae), known locally as agraz or mortiño, has raised its importance in the country as one of the exotic fruit crops of high value for national and international markets due to its high nutraceutical and medicinal properties (Maldonado et al., 2014; González et al., 2017). The plant has many uses including fresh fruit consumption, juices, jams, wines, ice cream or pastry, as well as the industry of beverages and processed food (Zapata et al., 2016; Lopéz et al., 2017). according to Garzón et al. (2010), the antioxidant effect of fruits of andean blueberry exceeds up to 3 times the raspberry fruits and other Rubus species, which is related to a high content of anthocyanins in its fruits (Montoya et al., 2012).
The genus Vaccinium comprises more than 450 species (abreu et al., 2014); in the andes several of these plants grow in open mountain slopes at altitudes between 2,3003,500 m a.s.l., so they are considered as sub-paramo and paramo plants (Chamorro and Nates-parra, 2015). In wild populations, the andean blueberry is presented as an evergreen shrub of 1-4 m size, with globose berries from 5 to 10 mm in diameter, purple-dark color when mature, and an edible slightly acid pulp. Ligarreto et al. (2011) recognized the andean blueberry as a promising species for cultivation in the country due to its high adaptability to different environments of the andean highlands. However, despite the productive potential of the andean blueberry, little research has been carried out on the mineral nutrition of this plant; this lack of information hinders a possible expansion and productive positioning of the andean blueberry crop in the country.
In general, the plants of genus Vaccinium, such as most calcifuge plants, are limited to acid soils (pH 4.2 to 6.5) characterized by high content of organic matter, high availability of iron and manganese, and the presence of ammonium (NH4+) as the predominant form of N in the soil solution (Korcak, 1988; Bryla and Strik, 2015). According to Maqbool (2013), the nitrification activity, as a conversion of NH4+ to NO3 -, is limited in soils where blueberries have evolved, and, consequently, most of the N available in soil is present as ammonium. Townsend (1967, 1969), Greidanus et al. (1972), and Peterson et al. (1988) agreed that fertilization of blueberries with NH4+ sources stimulates their growth, while supplying the plants with N as nitrate (NO3 -) induces leaf chlorosis and decreases growth. These symptoms seem to be related to a lower rate of absorption and/or assimilation of NO3 - compared with NH4+ (Merhaut and Darnell, 1995) considering that N is the element of the highest demand that Vaccinium plants require during the vegetative growth (Maqbool, 2013).
The plants absorbing NO3 - require a nitrate reductase enzyme, which is referred to be inefficient in blueberries (Merhaut and Darnell, 1996) to reduce NO3 - to NH4+ and metabolize it into amino acids that are used for protein synthesis (Salisbury and Ross, 2000). Studies conducted by peterson et al. (1988), Sugiyama and ishigaki (1994), Mer-haut and Darnell (1995) indicated that the uptake of NO3 - by roots of commercial Vaccinium sp. is limited compared to NH4+ due to the low activity of nitrate reductase (poon-nachit and Darnell, 2004). According to alt et al. (2017), the supply of NO3 - to the roots of V. ashei and V. corymbosum did not increase its transport in the xylem nor increased the nitrate reductase activity in leaves, which shows the presence of limitations in the absorption and translocation of NO3 - to the shoot. These limitations result in the reduction in growth of blueberries fertilized with NO3 -. Also, availability of carbohydrates at the time of nitrogen assimilation can have a marked effect on absorption and assimilation of N and blueberry growth (Merhaut and Darnell, 1996). In particular, N-deficient plants had increased carbon allocation towards formation of phenolic substances, such as anthocyanins (Bryant et al., 1983).
Currently, most studies on the andean blueberry assess the use of the fruits, plant propagation, and ecology (Chamorro and Nates-Parra, 2015, and references therein). According to ligarreto et al. (2011), in Colombia, a high genetic variability of the andean blueberry exists in wild populations; however the variation in size of fruits is low, which can be useful to use this species as a commercial crop. At the same time, to our knowledge, no published reports are available on fertilization plans or determination of nutrient requirements of the andean blueberry.
Considering that andean blueberry as well as blueberries (Vaccinium sp.) are established as commercial crops by cuttings and the further lack of information about nitrogen fertilization in the andean blueberry, this study was developed under the assumption that andean blueberry present a preference for using ammonia sources of N compared to NO3 - sources for its growth. The objective of the research was to evaluate the effect of mineral nutrition with different nitrogen sources (NH4+, NO3 - and their combination) on the content of pigments and vegetative growth of the andean blueberry.
Materials and methods
Plant establishment
Plant material was obtained from apical branches of adult andean blueberry (Vaccinium meridionale Swartz) plants grown in the greenhouses of the Faculty of agricultural Sciences of the Universidad Nacional de Colombia (Bogota, Colombia). The base of the cuttings was treated with rooting substance consisting of 0.5:4.5 w/w mixture of Hormonagro® (Colinagro, Bogota, Colombia) and kaolinite making a 400 mg kg-1 naphthalene acetic acid concentration in the rooting mixture. After the substrate mixing process, the cuttings were placed in plastic propagation trays of 8x8 cm alveoli using 3 cuttings per alveolus. The substrate used for rooting of cuttings consisted of a 1:1 w/w mixture of Klassman® (Klasmann-Deilmann, Geeste, Germany) peat without nutrients and quartz sand that was previously disinfected. The cuttings were placed on a greenhouse bench at low light conditions (70% black plastic mesh), where received mist irrigation twice a day for 5 min. During the rooting period the plants were not fertilized.
After 90 d, the rooted cuttings were collected with 7.95±0.19 cm length, 0.54±0.27 g fresh weight (FW), 7.14±2.91 leaves, and 8.43±3.55 cm2 leaf area (average ± standard deviation); the leaves were disposed directly over the cutting stem, so that the cuttings had no lateral shoots. The cuttings were transplanted into 15x20 cm black plastic bags, one cutting per bag, in the substrate consisting of a 5:1 v/v mixture of Klassman® peat without nutrients and quartz sand. The substrate was previously disinfected at a temperature of 105 oC for 6 h in an oven. The substrate had the following chemical characteristics before starting fertilization treatments: 1.3% total N, 0.02% total p, 0.61°% total Ca, 0.02% total K, 0.04% total Mg, 67.6 meq 100 g-1 CEC, pH 6.0, and 0.73 dS m-1 EC (saturated paste) (IGAC, 2006). The 0.5 cm layer of rice husk was placed on the substrate surface around each plant to inhibit the growth of algae.
The transplanted cuttings were positioned in plastic covered greenhouse having average values of 23°C (day) and 15°C (night) air temperature, 63% relative air humidity (Kestrel® 4000 Weather Meter, Nielsen-Kellerman Co, Boothwyn, USA), and 1193.5 μmol m-2 s-1 light conditions (quantum censor Lí-189, Li-Cor Inc., Lincoln, USA).
Fertilizer applications
Two days after transplanting into the bags, the application of nutrient solutions to cuttings via roots was initiated. Around 30 ml of the nutrient solution was applied 3 times per week and per plant to maintain the substrate humidity at a constant field capacity, according to the four treatments: 1) Control without N; 2) 50% NH4+ - 50% NO3 -; 3) 100% NO3 -; and 4) 100% NH4+.
During 148 d of fertilization the plants were treated with nutrient solution; no water was applied to the plants at any time. The concentration of N in the nutrient solution was 70 mg L-1, except the control treatment with 0 mg L-1 N. The nutrient solution contained other mineral elements in doses: 16 mg L-1 p, 40 mg L-1 K, 30 mg L-1 Ca, 24 mg L-1 Mg, and 0.1 mg L-1 Fe. These concentrations of N and other mineral elements were adapted from chemical composition of nutrient solutions used for growth of rooted blueberry cuttings under hydroponics conditions (poonnachit and Darnell, 2004; Tamada, 2004; Glonek and Komosa, 2013). The micronutrients, except Fe, were applied to the nutrient solution in quantity 1 ml L-1 according to the methodology followed Flórez and Cruz (2004). Distilled water was used to prepare the nutrient solutions.
As sources of mineral elements, pure salts of analytical grade were used: NH4N03, Ca(NO3)2, NaNO3, and Urea for nitrogen; CaCl2 for calcium; K2Hp04 for potassium and phosphorus; MgSO4 for magnesium and sulphur, FeSO4-7H2O for iron, and sources of other micronutrients according to Flórez and Cruz (2004). The sources of iron and other micronutrients were mixed with the rest of the nutrient solution immediately before applying to the plants.
A completely randomized design was carried out with 4 treatments; each treatment consisted of 35 plants at the start of the experiment. Five destructive samplings were carried out: 1st sampling: 2 d after transplanting (dat) (the moment when fertilizer application started); 2nd sampling: 42 dat; 3rd sampling: 77 dat; 4th sampling: 111 dat; 5th sampling: 148 dat. During each sampling 7 plants were randomly removed in each treatment to obtain direct and indirect growth variables.
The following direct variables were quantified on each 7 plants per treatment: number of shoots (primary branches originated at the cutting stem) and leaves; leaf area using leaf area meter LI 3100C® (LI-COR Inc., USA); dry weight (DW) of leaves, shoots, and adventitious roots obtained after drying samples at 65°C for 48 h; relative chlorophyll contents in leaves using SPAD® 502 (Minolta, Japan) on leaf limb, in 3 leaves of each cutting; contents of monomeric total anthocyanins in leaves (mg 100 g-1 FW) taking 3 leaves per each plant and using the differential pH method (extraction with 1% HCL in methanol, dilution in buffers pH 1.0 and pH 4.5 and spectrophotometry according to Lee et al. (2005)). The anthocyanin contents were measured using λ=530 nm (Spectrophotometer Spectronic® 501, Milton Roy, USA) and were calculated as cyanidin-3-glucoside equivalents (molecular weight of 449.2 g mol-1).
Growth indexes as indirect variables of plant growth were calculated according to Hunt (2013): Leaf area index (LAI) = LA/GA; Specific leaf area (SLA, cm2 g-1) = LA/LW; Net assimilation rate (NAR, g m-2 d-1) = (1/LA)x(dSDW/dt), where LA - leaf area (cm2), GA - area of soil surface; Lw - leaf DW (g), SDW - DW of shoots + leaves; t - time (d).
Results and discussion
Contents of chlorophyll and anthocyanins in leaves
The relative chlorophyll contents in leaves, for treatments 100% NH4+ and 50%NH4+ - 50%NO3 -, increased throughout the experiment and corresponded to 45.94 and 54.88 SPAD units at 148 dat, respectively (Fig. 1A). The plants treated with 0% N and 100% NO3 - at 148 dat had significantly lower chlorophyll levels in leaves of 20.44 and 21.40 SPAD units, respectively (Fig. 1A). A lower synthesis of chlorophyll in these treatments can be attributed to the preference of the Andean blueberry for ammonia forms of N and progressed N deficiency in the treatment of 0% N. For anthocyanin synthesis, the opposite trend was observed, where the treatments with 0% N and 100% NO3 -accumulated significantly higher contents of anthocyanins in leaves at the end of the experiment, with 12.79 and 11.68 mg 100 g-1 FW, respectively (Figs. 1B and 2). This behavior could be an adaptive response of N-deficient plants to protect leaves with low levels of chlorophyll (Fig. 1A) from the excess of light (Salisbury and Ross, 2000).
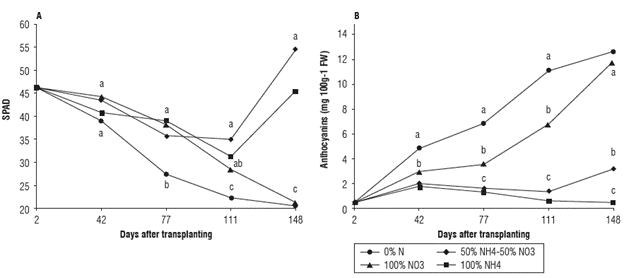
FIGURE 1 A) Relative chlorophyll contents (SPAD units) and B) Anthocyanin contents (mg 100 g-1 FW) in leaves of the Andean blueberry treated with different sources of N. Means followed by the different letters at each sampling date are significantly different according to Tukey test (P<0.05).

FIGURE 2 Plants of the Andean blueberry fertilized with A) 0% N, B) 100% NO3 -, C) 50%NH4+-50%NO3 -,or D) 100% NH4+at 148 dat.
Deficiencies of mineral nutrients can cause accumulation of anthocyanins in leaves of Vaccinium sp. (Routray and Orsat, 2011). According to Salisbury and Ross, (2000), N deficiency regulates routes of secondary metabolism in plants, such as synthesis of phenylpropanoids including flavonoids and anthocyanins. Bryla and Strik (2015) affirmed that plants of V. corymbosum deficient in N presented poor growth, with leaves that turned pale green or yellow and developed a reddish tinge. Leitzke et al. (2015) found that fertilization of blueberry with ammonium sulfate induced a higher synthesis of chlorophyll, and that high doses of the same source (more than 7.37 g per plant at reproductive stage), can negatively influence its growth and production. The same authors indicated increased contents of anthocyanins in plants subjected to abiotic or biotic stress; these effects were attributed to an excessive accumulation of N in leaves or a reduction of the soil pH and an increased availability of aluminum in soil when fertilized with ammonium sources of N (Leitzke et al., 2015).
Accumulation of anthocyanins in blueberry fruits contribute to their high commercial attractiveness due to their nutraceutical effects (Li et al., 2017); however, in the present study, the accumulation of anthocyanins in leaves was, evidently, an adaptive response to stress associated with a reduced production of chlorophyll. At the same time, blueberry leaves are used in food industry as sources of antioxidants due to their high content of anthocyanins (Ferlemi and Lamari, 2016). The leaves of Andean blueberry are used in beverage industry (Zapata et al., 2015), therefore, an addition of NO3 - to fertilizer formulas could be a practice aimed to increase anthocyanin contents in leaves for this purpose.
Number of leaves and leaf area
The number of leaves and leaf area progressively increased over time, where the treatment with 100% NH4+ reached a maximum of 254 leaves per plant (Fig. 3A) and the treatment of 50%NH4+ - 50%NO3 - reached the maximum 130 cm2 of leaf area (Fig. 3B) at 148 dat. Determination of leaf area is important to characterize crop growth, its potential yield, and efficiency in use of solar radiation, water, and mineral nutrients (Williams and Martinson, 2003). The growth rate of leaves is affected by expansion of young cells, which are produced by cell division in the meristematic tissues (Salisbury and Ross, 2000). For this reason, inadequate supply of N could diminish rates of leaf growth as it was evidenced in the treatments with only NO 3 - sources, where the plants of Andean blueberry generated fewer leaves and lesser leaf area (Fig. 3) due to the preference of various Ericaceae to NH4+ sources of N (percival and privé, 2002). The plants treated with 0% N and 100% NO3 - obtained maximum 50 and 119 leaves at 148 dat, respectively. The plants of 0% N treatment had poor leaf emission throughout the experiment and presented leaf abscission at 148 dat due to the lack of N supply, apparently, exhausting N reserves of cutting stem.
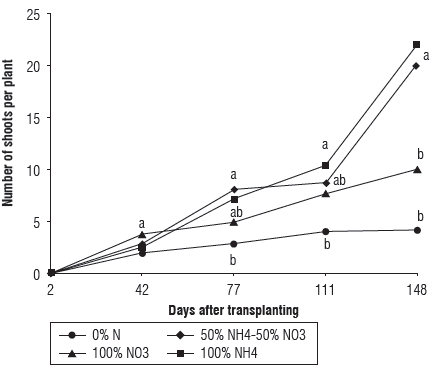
Number of shoots
The plants of Andean blueberry fertilized with NH4+ sources responded with a higher production of shoots (i.e. primary branches) on the cutting stem compared to the plants of NO3 - fertilization (Fig. 4). The number of shoots per cutting progressively increased over time, with the treatment of 100% NH4+ reaching maximum values of 22 shoots per plant followed by the treatment 50% NH4+ - 50% NO3 -, with 20 shoots per plant. During the evaluation period, control treatment 0% N had always the lowest number of new shoots (Fig. 4) as compared with other treatments. According to Takamizo and Sugiyama (1991), higher concentrations of N were recorded in shoots of blueberry plants fertilized with NH4+ with respect to those fertilized with NO3 -; the higher concentration of N, in turn, could contribute to formation of new shoots.
Dry weight of leaves, shoots, and roots
The highest leaf weight was registered in treatments 100% NH4+ and 50%NH4+ - 50%NO3 -(1.85 and 1.37 g DW, respectively) at 148 dat, while the other treatments had less than 1 g DW of leaves per plant (Fig. 5A). The accumulation of dry matter in leaves is directly related to the number of shoots and new leaves and to photosynthetic capacity that allows producing photoassimilates and developing higher leaf area. The plants supplied with 100% NO3 -, apparently, managed to assimilate some fraction of NO3 - into the cell proteins and surpass the treatment with 0% N, which counted up mainly on N reserves of cutting stem/roots to emit few leaves. This agrees with the data reported for blueberry by Merhaut and Darnell (1996), who affirm that, although the plants of Vaccinium sp. can absorb both NH4+ and NO3 - under the range of pH conditions optimal for growth, assimilation of NO3 -by these plants is limited as compared to NH4+. It should be noted that, in our experiment, the differences among the treatments in plant growth might be, at least in part, influenced by the changes in substrate pH that depended on the source of N used for the application.
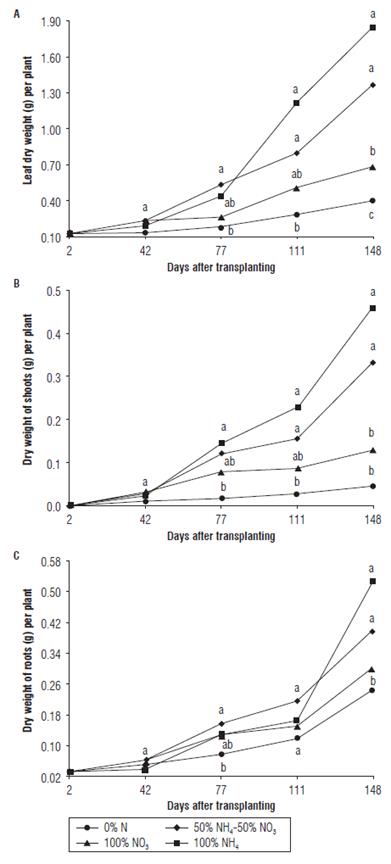
FIGURE 5 Dry weight of leaves (A), shoots (B) and roots (C) of the Andean blueberry treated with different sources of N. Data means followed by the different letters at each sampling date are significantly different according to the Tukey test (P<0.05).
Root DW in the treatments 100% NH4+ and 50%NO3 - -50%NH4+ increased over the time of the experiment (Fig. 5C); these plants exhibited the highest values of 0.52 and 0.40 g root DW per plant, respectively, at 148 dat. This correlates with the superiority of the same treatments in emission of leaves and shoots (Figs. 4 and 5) and agrees with Merhaut and Darnell (1995), who affirmed the lower assimilation rate of NO3 - in Vaccinium sp in comparison with that of NH4+. Birkhold and Darnell (1993) indicated that V. ashei during vegetative growth had a high concentration of N in both shoots and roots, since fast-growing organs strongly demand this element. The roots can serve as the storage site for N, which would be later translocated to new growing organs, even though the N concentration was adequate in the nutrient solution (Birkhold and Darnell, 1993). In another deciduous species as V. corymbosum, the highest accumulation of biomass during vegetative stage of growth corresponded to a period of maximum N absorption, with new emerging leaves being the organs of main demand for N (Fang et al., 2017).
Plant strategies to adapt for different types of stress frequently consist in decreasing leaf expansion, increasing root growth, or promoting the leaf abscission (Yepes and Buckeridge, 2011). The distribution of DW between the organs at 148 dat showed that plants of 0% N and 100% NO3 -assigned a higher proportion of biomass to formation of roots as compared with the treatments with NH4+ (Tab. 1).
TABLE 1 Distribution of dry weight among organs in plants of the Andean blueberry treated with different sources of N, % total dry weight of the plant (stem+shoots+leaves+roots).
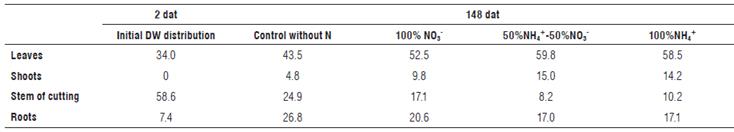
This could indicate that the plants searched for N sources to compensate N deficiency when growing without NH4+ supply. Additionally, the plants of Andean blueberry, in the absence of NH4+, expanded leaf area through emitting new leaves but invested much less resources in the formation of new shoots as compared to plants supplied with NH4+ (Tab. 1; Fig. 2).
Growth indexes
Leaf area index (LAI) expresses the leaf area over the surface area occupied by the plant (Hunt, 2013). The LAI of plants treated with N had an ascending behavior over the time (Fig. 6A) and at some point would reach a maximum value, at which the plants will have the maximum capacity to intercept solar energy (Hunt, 2013). The ascending behavior of LAI can be attributed to a high rate of leaf formation and foliar expansion, especially in treatments 100% NH4+ and 50%NH4+ - 50%NO3 -. The maximum values of LAI were not observed (Fig. 6A) because the moment of evaluation was limited to 148 dat of vegetative growth; the highest LAI of 1.06 was recorded at 148 dat for the treatment 100% NH4+. In treatment with 0% N, LAI did not exceed 0.2. Therefore, in plants of 100% NO3 - and, especially, 0% N, the behavior of LAI can be explained by the deficiency of N, with a consequent reduction in leaf area and a reduction in the proportion of dry mass accumulated in leaf tissues (Curtis and Láuchli, 1986). This deficiency might have progressively reduced photosynthetic rate in these plants due to a lower chlorophyll content accompanied by an increased production of anthocyanins (Fig. 1) and, in some cases, caused leaf senescence. According to behavior of LAI, the treatments with NH4+ could be considered as the best treatments. These plants produced the highest number of leaves (Fig. 3A) and shoots (Fig. 4) that allowed them a better interception of light that, consequently, would be reflected in higher production of photoassimilates and, therefore, allowed a greater accumulation of biomass in comparison with treatments of 100% NO3 - and 0% N.
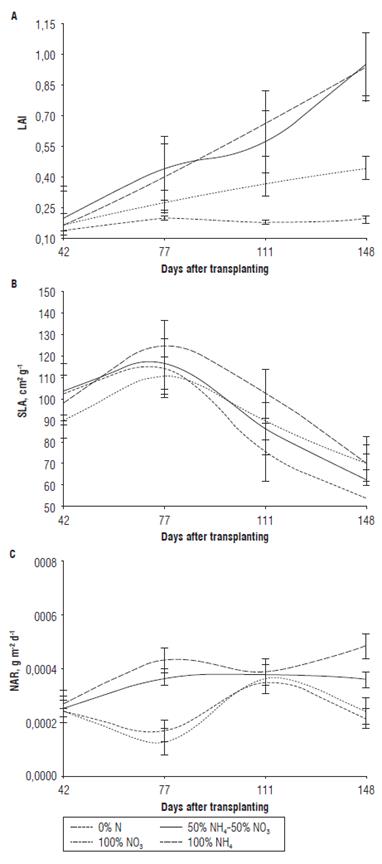
FIGURE 6 A) Leaf area index (LAI), B)Specific leaf area (SLA), and C)Net assimilation rate (NAR) of Andean blueberry plants treated with different sources of N. Vertical bars indicate standard deviations.
Specific leaf area (SLA) is defined as a ratio between the total leaf area of the plant and dry weight of leaves (Hunt, 2013). The tendency of SLA in all treatments was to increase between 42 and 77 dat (Fig. 6B), which can be explained by a higher increase in leaf area as compared to the increase in leaf DW. In this period, the increase of SLA could be explained by the expansion of leaf area in pre-existing leaves and new leaves, such as in 50%NH4+ - 50%NO3 - treatment. Apparently, this period of growth between 42 and 77 dat was characterized by increasing leaf surface, while the leaves maintained a low amount of mesophyll layers. The SLA increase in 0%N treatment from 42 to 77 dat could be due to consumption of photoassimilates/starch available in leaves/stem of cutting, which, in turn, might have reduced leaf thickness. Also, the marked decrease in SLA in 0% N treatment starting from 77 dat until the end of the experiment (148 dat) is attributed to the poor formation of new leaves (Fig. 3A), because the plants were depleting their N reserves. In other treatments, the decrease in SLA between 77 and 148 dat can be explained by a lower expansion of leaf area and increased transport of photoassimilates and/or N towards the leaves, which implied an increase in weight/ thickness of leaves. This behavior is common in deciduous blueberries, where an adequate supply of N results in N storage and subsequent remobilization for growth of new leaves and shoots (Birkhold and Darnell, 1993).
The net assimilation rate (NAR) is defined as the increase in plant dry weight per unit of leaf area and time (Hunt, 2013) and indicates average photosynthetic efficiency of plants. In plants treated with 100% NH4+ or 50%NH4+ -50%NO3\ NAR reached the highest values starting from 77 dat because, in this period, the plants were emitting new leaves (Fig. 3A). The treatments with NH4+sources presented the highest values of NAR because, as shown by LAI, these plants had the maximum leaf growth allowing the highest photosynthetic efficiency as well as the largest accumulation of dry matter.
In plants treated with 100% NO3 -or 0% N, the decrease in NAR between 42 and 77 dat (Fig. 6C) indicated a reduced photosynthetic efficiency in this period, which can be attributed to the reduction in activity of Rubisco and/or other proteins associated with the Calvin cycle (Salisbury and Ross, 2000). It can be hypothesized that an atypical increase in NAR between 77 and 111 dat for treatments 0% N and 100% NO3 - was due to retraslocation of N reserves preserved in the stem of cuttings towards the leaves. Between 111 and 148 dat, in treatments 0% N and 100% NO3 -, NAR decreased due to the senescence and abscission of leaves indicating N deficiency. In general, this study indicated that the Andean blueberry during vegetative growth, similarly to commercial blueberries Vaccinium sp, requires fertilizers with ammonia sources of N.
Conclusions
The effect of the nitric and ammonia fertilization on the growth of Andean blueberry was demonstrated showing that at vegetative stage of growth the plants had a higher accumulation of dry matter, higher number of shoots and leaves with application of NH4+ as N source. The plants subjected to NO3 - fertilization presented an increased production of anthocyanins in leaves due to the stress caused by nitrogen deficiency and associated with low synthesis of chlorophyll.





![Post-harvest quality of pineapple guava [Acca sellowiana (O. Berg) Burret] fruits produced in two locations at different altitudes in Cundinamarca, Colombia](/img/pt/next.gif)