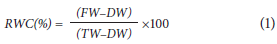Introduction
In recent years, the consumption of spinach (Spinacia olerácea L.) has increased worldwide. This vegetable provides a balanced supply of vitamins and minerals to diets, preventing many diseases (Jiménez et al., 2010). In Colombia, the cultivated area of spinach is close to 500 ha (MADR, 2017), and it is considered a crop with great export potential for markets in the United States and other consuming countries.
Water scarcity is one of the main consequences of climate change, which is one of the most important constraints for crop development (Farooq et al., 2009) since it generates economic losses and threatens global food security (Earl and Davis, 2003). Spinach is susceptible to drought since this condition reduces photosynthesis as a result of stomatal closure, which prevents the entry and assimilation of CO2. This effect may be reversible (acclimatization) or irreversible (damage to photosynthetic metabolism) (Lipiec et al., 2013).
The damage caused to plants by a water deficit depends on the duration and intensity of the water stress. Severe stress can reduce growth and absorption of nutrients such as phosphorus and nitrogen by up to 30%, while mild stress does not significantly affect these variables. A plant's capacity of recovery depends on both the intensity of the stress condition and the genotype (Subramanian et al., 2006).
Plants exposed to a water deficit present slow growth and a low photosynthetic rate, which is affected mainly by a decrease in the cellular turgidity and in the assimilation of CO2, which in turn causes lower accumulation of dry mass and poor plant development (Lipiec et al., 2013; Batra et al., 2014). In addition to these processes, variables related to water status in plants such as water potential, relative water content and osmotic potential have been reported as being sensitive to water deficits. Therefore, these variables are generally used as water deficit indicators (Lipiec et al., 2013).
Although water deficit is one of the more studied abiotic stresses, there are few studies on spinach, and there is no information on the recovery capacity of this plant and the main variables that are affected by water deficits. Since the importance of spinach is growing and its demand for water can be considerable, the study of this abiotic stress should be addressed. Therefore, the objective of this research was to evaluate the effect of water deficit on spinach growth, water status and plant production.
Materials and methods
This project was carried out in the mesh house of the Universidad Pedagógica y Tecnológica de Colombia (UPTC) in Tunja, located at an altitude of 2,782 m a.s.l. with coordinates 5°32' N and 73°23' W. The mesh house had a plastic cover, and the average temperature during the study was 16°C with 70% relative humidity (RH).
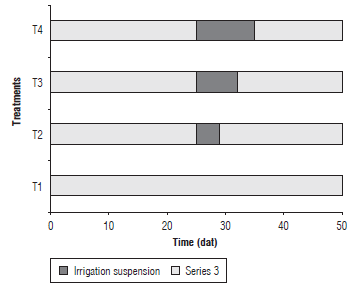
Hybrid Select 4-24 spinach seedlings, 20 d after germination, were transplanted to 2 L pots with a mixture of peat and soil (2:1). A completely randomized design (CRD) was used with four treatments (Fig. 1) corresponding to the duration of the irrigation suspension: T1: 0 d, T2: 4 d, T3: 7 d and T4: 10 d, with six replicates for a total of 24 experiment units, each one consisting of 6 plants for a total of 120 evaluated plants in the test. The irrigation was suspended according to the established treatments, after which the water supply was resumed. The sampling was done at 25, 29, 32, 35 and 50 d after transplanting (dat), and the growth variables were determined as detailed below.
The accumulation of fresh leaf mass was determined using a VIBRA AJ220E (Shinko Denshi Co, Tokyo, Japan) 0.001 g semi-analytical balance; for dry mass accumulation, the leaves were dried in a Memmert UNB500 drying oven (Memmert GmbH + Co. KG, Schwabach, Germany) at 80°C for 48 h. The leaf area and canopy leaf area were determined by taking photographs of the complete leaves and the canopy of the plant, respectively. These photos were analyzed with ImageJ (University of Wisconsin, USA).
The relative water content (RWC) was determined using 8 discs per leaf (1 cm in diameter) and the following equation (Smart and Bingham, 1974):
where FW is the simple fresh weight, DW is the leaf dry weight and TW is the turgid weight. FW corresponded to the mass of the discs immediately after cutting them from the leaf, TW corresponded to the mass of the discs after subjecting them to a relative humidity close to 100% for 24 h, and DW corresponded to the mass of the discs after drying at 80°C for 48 h.
The stomatal conductance was determined using a SC-1 meter (Decagon Devices Inc., Pullman, WA, USA). The relative content of chlorophyll was determined with a SPAD-502 Plus meter (Decagon Devices Inc, Pullman, WA, USA). The volumetric water content in the substrate was measured with a portable FieldScout TDR 100 meter (Spectrum Technologies, Aurora, IL, USA), and the water potential was determined at 12 m with a Scholander PMS 600 (PMS Instrument Co, Albany, OR, USA) on leaves that had been previously enfolded. A statistical analysis of variance and Tukey's comparison tests (P≤0.05) were carried out using the SAS v9.2e statistical program (SAS Institute Inc., Cary, NC, USA).
Results and discussion
Volumetric water content (VWC) in the substrate
There were significant differences between the treatments (Tab. 1). The control treatment (without irrigation suspension) and the treatment with 4 d of irrigation suspension reached the maximum values of VWC in the substrate (65.4%) at a depth of 4 cm at 32 and 35 dat, respectively. When analyzing the moisture behavior over time, it was observed that the VWC decreased as the suspension of irrigation progressed until 35 dat (Fig. 2), when the water supply was resumed, after which the soil moisture increased and remained almost constant until the day of harvest.
TABLE 1 Anova F values of the variables evaluated In the spinach plants submitted to different levels of water deficit.
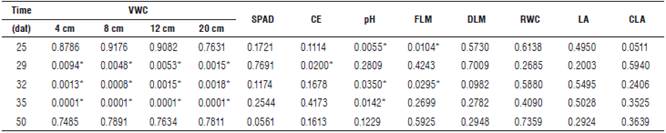
dat: days after transplating; VWC: Volumetric water content; SC: Stomatal conductance; FLM: Fresh leaf mass; DLM: Dry leaf mass; RWC: Relative water content; LA: leaf area; CLA: canopy leaf area. * Indicates significant differences at P≤0.05.
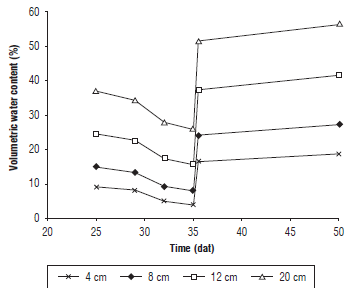
FIGURE 2 Behavior of the volumetric water content during the irrigation suspension in the substrate of the spinach plants.
As for the variation of the VWC in the substrate with respect to depth, it was observed that the upper part of the substrate maintained a higher moisture content, especially in the treatment without irrigation suspension and in the treatment with 4 d of irrigation suspension, probably because the water stress time was the lowest (Fig. 3). Durand et al. (2016) stated that roots extract water from the zone of contact with the medium; if there is a certain level of water deficit, the VWC of the substrate decreases, which can slow down or even stop the water absorption rate in plants because of the energy used for water absorption.
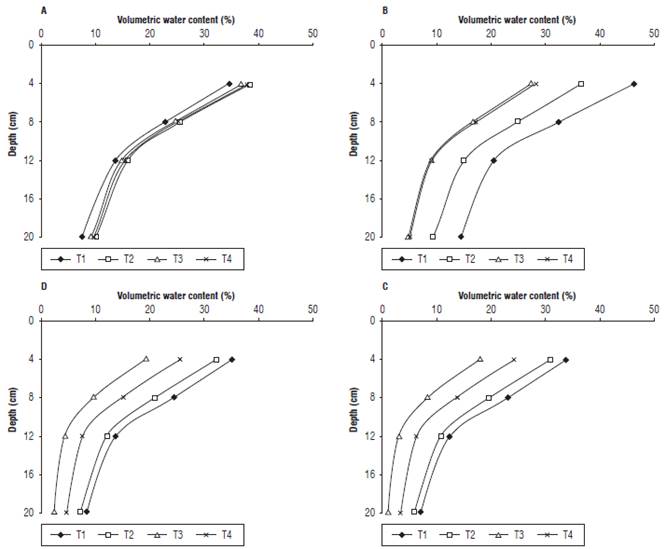
FIGURE 3 Volumetric water content in the substrate of the spinach plants with respect to the substrate depth, A: 25 dat; B: 29 dat; C: 32 dat; D: 35 dat.
Water stress causes a decrease in the synthesis of auxins and, in turn, an increase in ethylene synthesis. This increase in endogenous ethylene is slight, but sufficient to initiate the process of leaf abscission. In particular, the expression of genes encoding polygalacturonase, cellulase and aminocyclopropane-1-carboxylic acid synthase (ACS), the latter being the catalyzer of ethylene synthesis, has been found in the organ abscission zone (Estornell et al., 2013). Furthermore, to avoid water deficit problems, the recommended VWC value for a medium varies between 24% and 40% (Abad et al., 2004); this value depends on the physical characteristics (Bougoul and Boulard, 2006).
Chlorophyll content (SPAD)
There were no significant differences in the chlorophyll content between the plants submitted to different irrigation suspensions. The values showed a downward trend over time for the SPAD units, which ranged from 43.4 for the treatment with 10 d of irrigation suspension on day 25 (dat) to 28.4 SPAD units for the treatment with 4 d of irrigation suspension on day 50 (dat) (Tab. 1). The water deficit to which the plants were subjected was not intense and could not have affected the chlorophyll content. La Rosa et al. (2011) stated that, in spite of subjecting plants to certain levels of water stress, they did not present alterations in the synthesis of chlorophyll. In adittion, Ors and Suarez (2017) found values that ranged from 47 to 49 SPAD units for spinach plants subjected to different levels of water stress (soil water potentials -44.7 kPa, -231 kPa, and -446 kPa) and an electrical conductivity of 0.85 dS m-1.
Stomatal conductance
There were no significant differences between the treatments except for day 29 (dat), where the treatment with 10 d of irrigation suspension showed an average value of 333.8 mmol m-2 s-1, which then decreased markedly during the evaluation period (Tab. 1). It should be emphasized that the reduction of stomatal conductance has protective effects, allowing water savings in plants and improving water use efficiency (Chaves et al., 2009). Plants have also generated responses to water stress with adaptations at the morphological, anatomical and cellular levels, which allow them to live in conditions of constant water stress. In this regard, the stomatal closure process acts as a resistance mechanism at the physiological level. It occurs when the mesophyll begins to undergo dehydration, which is regulated by abscisic acid (ABA), increasing its content in the leaves. This happens as a result of the decompartmentalization and redistribution of chloroplasts in mesophyll cells and its synthesis and transport from the roots, where it is released to the apoplast, reaching the guard cells through the transpiration stream (Moreno, 2009).
Water potential
This variable presented significant differences in the Anova test. An inversely proportional relationship was observed between the moisture content and the water potential of the leaves. The treatment without irrigation suspension registered the highest leaf water potential value during the measurement period, obtaining a value of -0.59 MPa for day 25 (dat) and a value of -0.91 MPa for day 34 (dat), whereas the treatment with the least water (T4: 10 d of irrigation suspension) recorded the lowest water potential values, -1.02 MPa for day 25 (dat) and -1.32 MPa on day 34 (dat) (Tab. 1).
On the other hand, these results are consistent with those reported by Godoy et al. (2005) and Ismail (2010), who indicated that, the lower the availability of water in a soil, the lower the water potential. In this regard, the substrate retained an amount of homogeneous water, and the evapotranspiration, infiltration and absorption by the plants resulted in water loss, which manifested a greater decrease in the moisture content in the treatments where the water stress lasted longer. It should be noted that the water potential values were lower than those reported for other crops, such as the faba bean (Kajerti et al., 2011), which ranged from 0.15 to 0.5 MPa because the leaf water potential in this research was measured at noon since the objective was to determine the maximum stress to which the plants were subjected in the different treatments.
Leaf area (LA)
No significant differences were found for LA; however, for the first LA measurement at 25 dat, irrigation restriction for 4, 7 and 10 d decreased the leaf area production by 6%, 10% and 26%, respectively. This indicates that increasing the water restriction of spinach is detrimental to leaf area development and decreases the moisture content in a substrate because, as the irrigation suspension increased, the substrate presented a lower moisture content at the all of the measured depths (Fig. 3). In this regard, Quintal et al. (2012) stated that a water deficit restricts cell growth, which leads to a smaller leaf expansion. Balaguera et al. (2008) indicated that, with a lower turgidity pressure resulting from a water deficit, the leaf area is smaller, and there is greater stomatal closure.
Dry mass and fresh mass of leaves
No significant differences were found between treatments in the dry mass, but there were significant differences for the fresh mass. The plants that were not submitted to irrigation suspension presented a greater recovery of bio-mass at 32 dat (Tab. 1). Quintal et al. (2012) indicated that, when there is a water deficit, leaves are less developed, and there is a smaller leaf area. This is why crop production correlates directly with the availability of water in the substrate. Reyes-Matamoros et al. (2014) concluded that water stress induces metabolic irregularities such as a decreased leaf growth rate and the consequent decrease in dry mass. These stress conditions have a strong impact on the morphology and physiology of plants, which depends on the degree of tolerance of the tissues to dehydration, mainly in leaves which are the photosynthetic surface. Furthermore, water stress affects the ability to accumulate solutes, which are required to maintain an adequate water content in plants to prevent diminished growth. A greater reduction in the production of fresh mass in spinach leaves and water use efficiency was also reported by Ors and Suarez (2017) when plants were subjected to a combined hydric and saline stress instead of receiving a separate effect from these two types of stress.
Relative water content (RWC)
For the relative water content, there were no significant differences between the treatments. The values ranged from 85.5% for the treatment that did not have irrigation suspension at 28 dat to 73.9% for the treatment with 10 d of irrigation suspension at 34 dat (Tab. 1). Bartlett et al. (2012) pointed out that a total relative water content in cells of less than 75% can drastically inhibit the production of adenosine triphosphate (ATP), Ribulose 1,5-bisphosphate (RuBP) and proteins; however, this depends on the resistance to water movement outside the cells and the water potential in the vascular bundles. Because of water deficits, the cell membrane undergoes changes, such as permeability and decreased turgidity (Blokhina et al., 2003). In addition, microscopic investigations of dehydrated cells revealed damage, including membrane rupture and sedimentation of cytoplasm content, which can reduce the ability of osmotic adjustment (Ganji Arjenaki et al., 2012).
Conclusions
The irrigation suspension for 10 d decreased the moisture content of the substrates in which the spinach crop was planted. The volumetric water content was higher in the first centimeters of the substrate and decreased with depth. The water potential of the spinach plants was affected by the irrigation suspension because, with the longer period without water, there was higher water stress, and the plants presented a lower leaf area and fresh mass. The leaf chlorophyll content of the spinach plants was not affected by the different irrigation suspension treatments.