Introduction
The world's growing population in recent years has been accompanied by increased food demand, and oleraceous crops are important to meeting this growing demand. In addition, oleraceous crops contribute directly to family farming activities (Noda and Noda, 2016).
The radish (Raphanus sativus L.) is an edible root vegetable of the Brassicaceae family, originated in the Mediterranean region, which presents globular swollen tap root with white or reddish skin. The radish is a fast-growing vegetable that can be harvested 25 to 35 d after sowing. The fast harvest cycle makes it particularly suitable for small and medium oleraceous producers, who can cultivate them along with longer cycle crops (Filgueira, 2008).
The radish contains significant amounts of vitamins A, C and B, and minerals such as calcium, phosphorus, magnesium, iron, sodium and potassium, which are essential nutrients to humans (Vidigal et al., 2007). The radish crop, like other oleraceous crops, requires quality water to fully grow.
Irrigated agricultural systems require water availability and quality. The occurrence of low precipitation rates and saline soil conditions in arid and semi-arid regions around the world prevents salts from leaching, causing their accumulation within soil and water. High salt concentration conditions cause nutritional imbalance, reduced transpiration and photosynthesis rates, lower leaf CO2 availability, and restricted stomatal conductance, which result in morphologically reduced plant biomass (Silva et al., 2013; Silva et al, 2018).
Salinity stress comprises two main components. The osmotic component, in which a high salt concentration in the soil solution reduces water availability to plants, and the ionic component, which is related to the toxicity of the ions released by the salts (Silveira et al., 2010; Prisco et al., 2017). Taking into account the necessity of using low quality water in irrigation systems, several studies have attempted to find ways to make these waters suitable for plant development and productivity (Oliveira et al., 2016; Rodrigues et al., 2017; Veras et al, 2018).
Ascorbic acid is a major antioxidant found in plants and plays an important role in several cellular processes. The foliar application of ascorbic acid stimulates photosynthetic pigment production and plant growth under stress conditions. Several studies have indicated that ascorbic acid plays a fundamental role in protecting plants from multiple environmental stresses, including salinity stress (Maia et al., 2012; Melo et al, 2014).
In the literature, there are several reports on the use of ascorbic acid for attenuating the deleterious effects of saline stress, such as in Triticum aestivum (Abbasi and Fakhani, 2015; Siddiqui et al., 2018), Zea mays (Billah et al, 2017), Linum usitatissimum (El-Bassiouny and Sadak, 2015), Citrus aurantium (Kostopoulou et al, 2015), and Hordeum vulgare (Agami, 2014). Based on this, the aim of this study was to evaluate the mitigating effect of ascorbic acid on the morphophysiological characteristics of salt stressed radish (Raphanus sativus L.).
Materials and methods
This experiment was carried out from October to November, 2017 in a greenhouse at the Agricultural Sciences Center of the Federal University of Paraiba (UFPB), located in the city of Areia, Paraiba, Brazil. The soil used in the experiment was collected in the 0-20 cm depth from the Plant Science Department land in the Federal University of Paraiba. This soil was classified as Latossoil (EMBRAPA, 2014), and its chemical analysis is shown in Table 1.
TABLE 1 Soil analysis report.

OM - organic matter, BS - base sum, CEC - cation exchange capacity, BSA - base saturation.
The radish variety used was the hybrid Margaret Queen Kobayashi (ISLA®). Five seeds were sown per plastic bag at a depth of 2 cm, and emergence occurred 5 d later. Then, the treatments with the saline water irrigation, according to the predetermined ECw values, were initiated. Ten d after emergence, the plants were thinned to one plant per bag.
The experimental design was a randomized block with the treatments distributed in a 5x5 factorial scheme, generated with the Central Box experiment matrix (Bortoluzzi and Alvarez, 1997). The factorial design was composed of five electrical conductivities of the irrigation water (ECw): 0.50, 1.30, 3.25, 5.20 and 6.00 dS m-1, and five ascorbic acid (AA) doses: 0.00, 0.29, 1.00, 1.71 and 2.00 mM, using four replicates. Plastic bags with a 3.0 dm3 capacity, containing one plant each, represented the experiment units.
The water with the ECw of 0.50 dS m-1 was obtained from the UFPB water supply system. A mixture of NaCl, CaCl2.2H2O and MgCl2.6H2O at a 7:2:1 ratio was added to the 0.50 dS m-1 water to prepare the saline treatment water with the higher ECw values (1.30, 3.25, 5.20 and 6.00 dS m-1) (Medeiros, 1992). A micro processed portable conductivity meter CD-860 (Instrutherm, Brazil) was used to prepare the saline waters. Irrigation management was measured using a drainage lysimeter (Alves et al., 2017).
The radish leaves were sprayed with ascorbic acid until completely wet 14 d after seedling emergence. The non-ionic surfactant Tween 80® was added to the ascorbic acid solution for a better absorption effect at a concentration of 0.0002%.
At 15 and 30 d after seedling emergence, the following development variables were evaluated: shoot height, measured from base to leaf apex using a graduated ruler in centimeters; leaf number, counting all the leaves from each plant; tuberous root diameter determined at 2 cm of the soil using a digital pachymeter; chlorophyll a, chlorophyll b, total chlorophyll content and chlorophyll a/b ratio. The chlorophyll contents were determined with a portable electronic chlorophyll meter model CFL 1030, ClorofiLOG® (Falker, Brazil), and one reading was performed on each plant in totally expanded leaves.
The initial fluorescence (F0), maximum fluorescence (Fm), variable fluorescence (Fv) and quantum yield of photosystem II (Fv/Fm) were measured at 28 d after seedling emergence. Chlorophyll fluorescence measurements (F0, Fm, Fv and Fv/Fm) were obtained using the modulated fluo-rometer Plant Efficiency Analyser - PEA II® (Hansatech Instruments Co., UK), and the readings were performed between 09:00 and 10:00 in the morning, on young, fully expanded, non-harmed and well lighted leaves. The fluorescence parameters were measured after a period of 30 min of dark adaptation with foliar tweezers (Konrad et al., 2005). The obtained data were submitted to analysis of variance and regression using the software R (R Core Team, 2017).
Results and discussion
It was observed that the saline irrigation water had an effect on the development variables assessed at 15 and 30 d after seedling emergence. The shoot height, leaf number and tuberous root diameter were reduced with the increasing ECw values (Fig. 1). Differences between the two assessment periods on the above-mentioned variables were observed once they were development variables, and over a period of 15 d there was an increase in size.
Aerial development is an important characteristic in salt stressed plant evaluation because it provides important information on plant response to stressful conditions (Jamil et al., 2007). For the aerial part development variables, shoot height (Fig. 1A) had a higher value than the one predicted within the confidence interval in the electrical conductivity of 5.20 dS m-1, with plants reaching a mean value of 10.5 cm. At the electrical conductivity of 6.00 dS m-1, the radish plants reached an average value of 8 cm. Different results were observed by Putti et al. (2016), who registered no significant difference in radish behavior when submitted to 5.00 dS m-1 level of salinity.
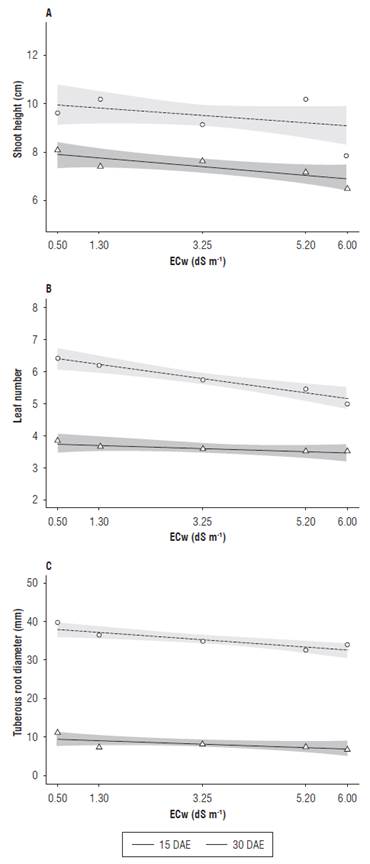
FIGURE 1 Shoot height (A), leaf number (B) and tuberous root diameter (C) of radish (Raphanus sativus L.) subjected to different electrical conductivities of the irrigation water (ECw) at 15 and 30 DAE.
At 15 DAE, the leaf number (Fig.1B) remained constant at all ECw levels. At 30 DAE, the leaf number decreased as the salinity increased. A similar result was found by Nascimento et al. (2015), who registered reduced leaf numbers in pepper (Capsicum annum L.) subjected to increasing salinity levels. Reduced leaf growth is a plant defense mechanism under stress conditions, such as water deficit and salinity, and it diminishes transpirational water loss (Taiz et al., 2017). These results showed leaf sensitiveness to salinity stress, which made them reduced both in size and number as the salt concentration increased in the irrigation water.
For tuberous root diameter (Fig. 1C), a slight reduction was observed as the concentration of salts in the irrigation water increased at 15 and 30 DAE. The results obtained in this study corroborate those obtained by Bacarin et al. (2007), who studied the salinity effect on the growth and development of radish grown in a nutrient solution, and verified a reduction in leaf area and tuberous root diameter in response to the salinity stress.
Figure 2A shows shoot height as a function of the ascorbic acid treatment. The highest values were 7.8 cm at 15 DAE and 10.6 cm at 30 DAE, obtained in the absence of AA and at the dose of 1.71 mM, respectively. The lowest shoot height values were observed at the highest AA dose (2.00 mM), with 7.3 and 8.8 cm at 15 and 30 DAE, respectively.
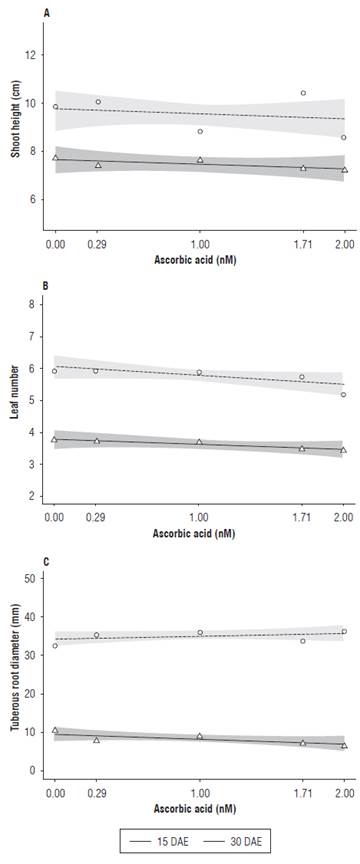
FIGURE 2 Shoot height (A), leaf number (B) and tuberous root diameter (C) of radish (Raphanus sativus L.) subjected to different ascorbic acid doses at 15 and 30 DAE.
For leaf number, a constant number of leaves was observed for all AA doses applied at 15 DAE. At 30 DAE, the radish plants treated with 0.29 mM of AA had a slight increase in the leaf number when compared to the control treatment plants (0.00 mM), showing that, as the concentrations of AA increased, a 13% decrease was observed in the leaf number, as compared to the control treatment (Fig. 2B).
Ascorbic acid is closely related to photosynthesis and respiration processes. The AA concentration in plant tissue varies as a function of plant response and different stress conditions, such as intensity and species sensitiveness to stress (Venkatesh et al., 2012). Plants that present a low ascorbic acid synthesis capacity are very sensitive to environmental stress conditions, thus their growth and development are affected and, in some situations, an exogenous AA application is necessary in order to supply this antioxidant deficiency (Gao and Zhang, 2008).
At 15 DAE, increases in the AA concentrations resulted in a slightly reduced tuberous root diameter. The control plants presented a 0.9 mm tuberous root diameter, whereas the radish plants treated with 2.0 mM of AA presented a 0.7 mm tuberous root diameter. Different results were observed at 30 DAE, when the control plants presented a 34 mm tuberous root diameter, and the plants treated with 2.0 mM of AA reached 38 mm (Fig. 2C).
The foliar a, b, total and a/b chlorophyll indices (FCI) were influenced by the addition of salts in the irrigation water (Fig. 3). In general, the plants subjected to the highest electrical conductivity of the irrigation water (6.0 dS m-1) and evaluated at 30 DAE showed the highest chlorophyll a, b and total values. For the assessment period, no differences were observed for the chlorophyll a or total chlorophyll. However, differences for chlorophyll b in electrical conductivities around 5.2 dS m-1 and higher were observed. For the chlorophyll a/b ratio, differences were only registered in the electrical conductivity of 3.25 dS m-1.
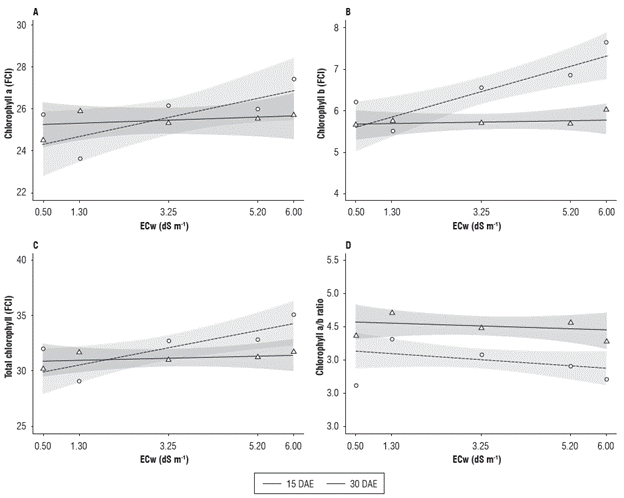
FIGURE 3 Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and chlorophyll a/b ratio (D) of radish (Raphanus sativus L.) subjected to different electrical conductivities of the irrigation water (ECw) at 15 and 30 DAE.
No difference was observed in the chlorophyll a content as a function of time; in the first evaluation (15 DAE), the lowest value (24.62) was found in the ECw of 0.50 dS m-1, and the highest value (25.91) was seen in the ECw of 1.30 dS m-1, with a decreasing response from this electrical conductivity. In the second evaluation period (30 DAE), the minimum and maximum values were 23.75 and 27.54 in the ECw of 1.30 dS m-1 and 6.00 dS m-1, respectively (Fig. 3A).
The chlorophyll b content (Fig. 3B) presented the highest values of 5.92 at 15 DAE and 7.68 at 30 DAE, both occurring in the ECw of 6.00 dS m-1. The assessment period influenced the chlorophyll b values in relation to the ECw treatment. When comparing the results obtained at 15 and 30 DAE, an increase for all tested ECw was observed, except for 1.30 dS m-1. These increments were 10.8, 15.1, 20.2 and 32.8% for the ECw of 0.5, 3.25, 5.20 and 6.00 dS m-1, respectively.
Figure 3C shows that the lowest value for the total chlorophyll content at 15 DAE was 30.2, obtained in the ECw of 0.50 dS m-1, while the highest value (32.1) was found in the ECw of 1.30 dS m-1. At 30 DAE, the lowest value was 28.8, obtained in the ECw of 1.30 dS m-1, whereas the highest value of 35.1 was observed in the ECw of 6.00 dS m-1. The chlorophyll a/b ratio presented higher values at 15 DAE, with a maximum and minimum value of 4.7 and 4.25, obtained in the ECw of 1.30 and 6.00 dS m-1, respectively. In the second evaluation (30 DAE), the highest value was 4.28, and the lowest was 3.67, obtained in the ECw of 1.30 and 0.50 dS m-1, respectively (Fig. 3D).
These increases in the plant chlorophyll content when submitted to higher ECw levels are contrary to those found by some authors (Chaparzadeh and Behboud, 2015; Jasim et al., 2016; Kasim et al., 2016). However, their studies were conducted with higher salinity levels, and they did not clarify the radish variety used. According to Jamil et al. (2007), the chlorophyll content is reduced in plants that are sensitive to salinity and increases in tolerant plants.
The content of chlorophyll a, chlorophyll b, total chlorophyll and the chlorophyll a/b ratio were affected by the ascorbic acid treatment. The content of chlorophyll a and total presented no difference in relation to the assessment period. For the chlorophyll b content, there was a difference between 1.0 and 1.71 mM of AA, whereas for the chlorophyll a/b ratio, there was a difference in the 0.29, 1.0 and 1.71 mM of AA (Fig. 4).
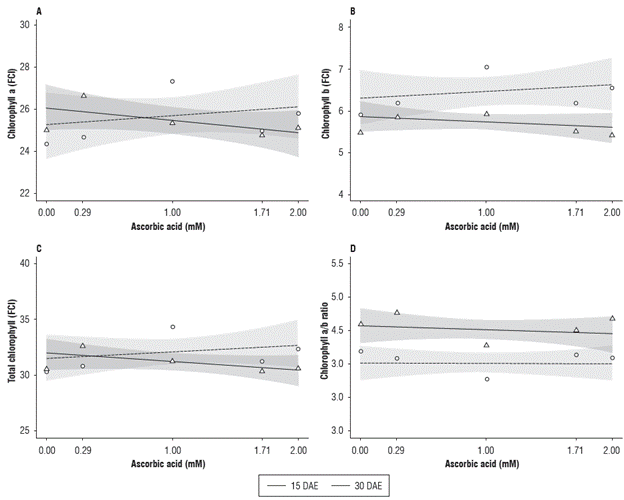
FIGURE 4 Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and chlorophyll a/b ratio (D) of radish (Raphanus sativus L.) subjected to different ascorbic acid doses at 15 and 30 DAE.
At 15 DAE, the maximum value of chlorophyll a was 26.83, obtained when submitted to 0.29 mM of AA. From this AA dose on, the chlorophyll a content decreased and reached a minimum value of 25.09 at the dose of 1.71 mM. At 30 DAE, the lowest chlorophyll a content value (24.47) was observed in the absence of AA, and the highest (27.62) was obtained with the AA dose of 1.00 mM, with decreasing values from this dose on (Fig. 4A).
Figure 4B shows that the chlorophyll b content was affected during the evaluation periods. At 30 DAE, the chlorophyll b values were higher, with increases in all AA doses in relation to the evaluation performed at 15 DAE, representing increments of 5.6; 11.3; 18.6; 16.3 and 19.2% for the 0.00; 0.29; 1.00; 1.71 and 2.00 mM of AA, respectively.
The total chlorophyll presented little variation in relation to the different doses of AA and the two assessment periods (Fig. 4C). At 15 DAE, the highest value was 32.52, obtained in the AA dose of 0.29 mM, and, from this dose on, the total chlorophyll content started to decrease, with 30.08 being the lowest value at the dose of 1.71 mM. At 30 DAE, the minimum value was 30.07, obtained in the absence of AA with a quadratic effect, and the maximum value was 34.65 with 1.00 mM of AA.
The lowest chlorophyll a/b ratios were observed in the AA dose of 1.00 mM, 4.28 and 3.79 at 15 and 30 DAE, respectively. The highest values for each assessment period were 4.78 at 15 DAE and 4.16 at 30 DAE, obtained with the doses of 0.29 mM and absence of AA, respectively (Fig. 4D). The chlorophyll content increase up to the 1.00 mM dose can be explained by the role of AA in regulating plant biochemical/ physiological reactions (Khan et al., 2006). However, higher doses of AA do not provide the same effects.
For initial fluorescence, it was observed that the maximum efficiency (93.6) was obtained under the electrical conductivity of 4.3 dS m-1. The maximum and variable fluorescence presented mean values of 428.25 and 342.68, respectively (Fig. 5A-C). Distinct results were found in the literature. When studying the NaCl effect, up to the concentration of 220 mM on salt-stressed radish plants, Bacarin et al. (2007) found no significant differences in the initial fluorescence as the salinity level increased.
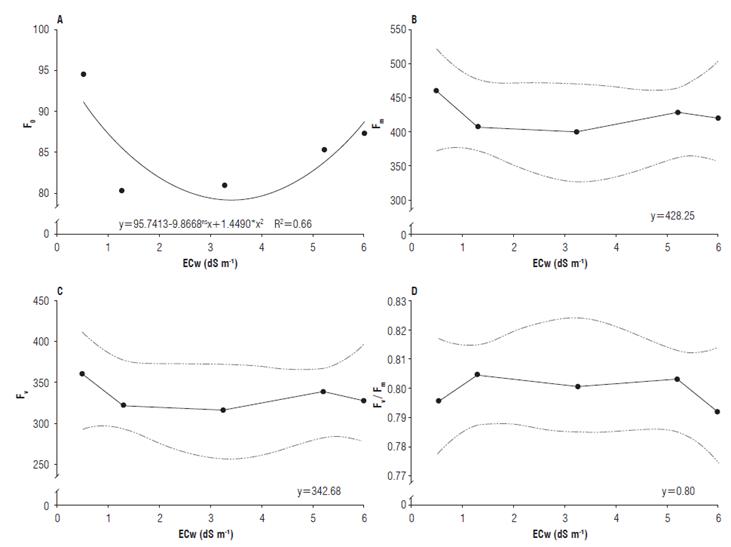
FIGURE 5 A) Initial fluorescence (F0); B) Maximum fluorescence (Fm); C) Variable fluorescence (Fv); and D) Quantum yield of photosystem II (Fv/Fm) of radish (Raphanus sativus L.) subjected to different electrical conductivities of the irrigation water (ECw).
For the quantum yield of photosystem II, the highest value (0.806) was obtained in the ECw of 1.30 dS m-1, while the lowest value was 0.792 in the ECw of 6.00 dS m-1 (Fig. 5D). Jamil et al. (2007) reported decreases in the quantum yield of photosystem II of radish plants as the salinity level increased.
Conclusions
The saline water influenced the analyzed variables in the radish (Raphanus sativus L.) plants regardless of the ascorbic acid application. The ascorbic acid was not efficient in attenuating the deleterious effect of salinity in the irrigation water on the development and fluorescence of the radish plants. However, the concentration of 1.00 mM of ascorbic acid promoted increased chlorophyll a, b and total in the salt-stressed radish plants.















