Introduction
Banana (Musa AAA) is the most important crop in Ecuador, which generates two million jobs and accounts for almost 10% of the total exports (Clúster Banano, 2018). In 2018, 345 million boxes of 18.14 kg were exported to the European Union, Russia, USA, Japan, China, and others, with a production area of 200,000 ha, which gave a total income of about US $2,700 million (Salazar, 2019).
Besides the requirements and demands of the banana market, there are other limiting factors to the production of this crop. Abiotic factors affecting yield, such as the edaphic soil condition, radiation, rain distribution, and temperature, are constraints to banana production in Ecuador. Among the biotic factors, banana-root nematodes are second after black Sigatoka caused by the fungi Pseudocercospora fijiensis. Banana nematodes live within the roots, where they weaken the plant anchorage and restrict water and nutrients uptake, retard leaf emission, and reduce photosynthesis, bunch weight, ratio, ratooning, and plant longevity (Gowen et al., 2005; Quénéhervé, 2008).
Plant-parasitic nematodes are common in the five provinces where banana is produced in Ecuador (Cañar, El Oro, Guayas, Los Ríos, and Santo Domingo) (Aguirre et al., 2016a; 2016b) and usually only polyspecific communities occur, consisting of a mixture mainly of Radopholus similis and Helicotylenchus spp. To avoid or reduce nematode damage, the only alternative management strategy currently available is the regular application of non-fumigant nematicides, due to its low cost for growers. Nematicide application is recommended when the total plant-parasitic nematode population exceeds the economic threshold of 2,500 individuals per 100 g of fresh roots (Instituto Nacional Autónomo de Investigaciones Agropecuarias INIAP, 2018).
The nematicides registered for bananas are rotated according to their physical-chemical characteristics and weather conditions to prevent their biodegradation. However, in Ecuadorian conditions, most banana growers stopped applying nematicides which resulted in high nematode populations, root damage, and severe yield reduction. In young plantations of less than 5 years old, the vigor of plants and their production, generally are higher than 3,000 boxes of 18.14 kg per hectare per year, make technicians and growers think that the attack of nematodes is negligible and that its effect on production is not of economic importance. Therefore, the objective of this study was to evaluate the effect of nematicide rotation in different applications per year on banana plant-parasitic nematode control and crop yield and to determine the net profit of the chemical nematode control in the crop.
Materials and methods
The field experiment was carried out in a 6-year-old renovated commercial banana (Musa AAA cv. Williams) farm infected by plant-parasitic nematodes located in the Milagro county, province of Guayas, Ecuador. The soil was taxonomically classified as an Inceptisol and it had a loamy texture (33% sand, 49% silt and 18% clay) with a pH of 7.1 and 1.8% organic matter. The following concentrations of extractable bases were found using Mehlich 3 (Mehlich, 1984) as the extractant: Ca 16.5, Mg 5.7, and K 2.4 cmol L-1, and P 29, S 16, Zn 3.1, Cu 7.6, Fe 60 and Mn 9 μg ml-1. The area where the experiment was established had an average production in 2015 of 3,600 boxes of 18.14 kg ha-1. The evaluation period was performed from October 2015 to October 2017.
Plant density was about 1,450 plants ha-1. De-suckering was carried out every eight weeks, leaving the production unit with a bearing mother plant, a large daughter sucker (follower) and a small grand-daughter (pepper) when possible. Bunching plants were propped with double polypropylene twine to the bottom of two well-developed adjacent plants, reason why plant toppling was not considered as a variable in the experiment. The follower sucker of each production unit was fertilized every 15 d with a mixture of nutrients at 100 kg ha-1, adapted to the soil and crop requirements, consisting of 15-4-36 (N-P2O5-K2O) fertilizers.
During the rainy season, from January to May each year, water requirements were supplied by rainfall. Annual precipitation was 1,771, 2,190 and 1,656 mm per year, for 2015, 2016 and 2017, respectively. A complex system of primary, secondary and tertiary drains was provided to disperse excess rainfall and prevent waterlogging during heavy rains. From June to December each year, water was supplied by sprinkling irrigation. Mean daily maximum/ minimum temperatures were 29-31/25-22oC during the studied period.
Leaf fungi, especially black Sigatoka (Pseudocercospora fijiensis), was managed by deleafing weekly to reduce the pressure of black Sigatoka inoculum and by aerial spraying of alternate fungicides which resulted in 24 sprayings per year with 8 to 14 d intervals. The fungicides applied were: difenoconazole, fenpropimorph, epoxiconazole, tebuconazole, isopyrazam+azoxystrobin, pyrimethanil, spiroxamine, metiram, mancozeb, Bacillus subtilis in emulsion with miscible oil (Spraytex) and water or in a water solution of 19 L ha-1. Weeds were controlled spraying every 12 weeks a glyphosate solution of 1.5 L in 200 L of water with a manual knapsack sprayer. Before the beginning of the experiment, nematodes were controlled every year by the rotation of one nematicide application (Verango® 50SC-fluopyram-Bayer, Vydate® 24SL oxamyl-DuPont, Counter® 15GR-terbufos-AMVAC).
Six treatments were evaluated: 1 and 2 consisted of two different nematicide rotations per year; 3 and 4 consisted of three different nematicide applications per year; 5: nematicide application based on nematode economic threshold of 2,500 plant-parasitic nematodes per 100 g of roots (INIAP, 2018), and 6: the untreated control. The applied nematicides were those available in Ecuador, including Counter® 15GR (terbufos-AMVAC), Verango® 50SC (fluopyram-Bayer), Vydate® 24SL (oxamyl-DuPont), Mocap® 15GR (ethoprophos-AMVAC) and Rugby® 10GR (cadusaphos-FMC) (Tab. 1).
TABLE 1 Description of the treatments evaluated with the sequence of nematicides and date of application.
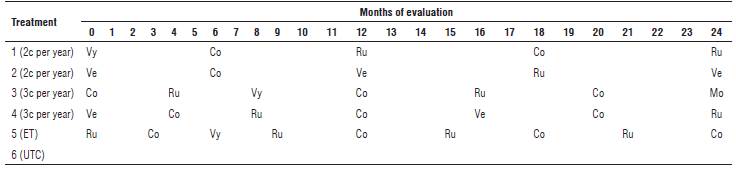
Note: month 0= October, 2015 and 24= October 2017. c per year= number of nematicide cycles per year. ET= Application based on the economic threshold of 2,500 nematodes per 100 g roots. UTC= Untreated control. Vy= 2.4 g a.i. Vydate® 24SL, Co= 3 g a.i. Counter®15GR, Ru= 2.0 g a.i. Rugby®10GR, Ve= 0.3 g a.i. Verango®50SC, and Mo= 3 g a.i. Mocap®15GR.
The rectangular plots for each treatment consisted of 150175 production units. Plots were arranged in a randomized complete block design with six replicates. The application was performed by spreading the products in a banded arc with a radius of approximately 0.40 m around each follower sucker pseudostem sprouting from the base of the sucker. The Swissmex backpack equipment specific for Counter®, Rugby®, and Mocap® and the spotgun for Vydate® were used for the application. The rates used per follower sucker were the recommended by the manufacturer: 3 g a.i. for Counter® and Mocap®, 2.4 g a.i. for Vydate®, 2 g a.i. for Rugby® and 0.3 ml a.i. for Verango®. Verango® was applied in a water solution adding 1 L of the product to 150 L of water plus 200 g of blue coloring, and 100 ml of this solution was spread onto the soil surface with a manual dosing snack pack. Plant debris was removed from the soil surface prior to distributing the nematicides onto moist soil as directed by the product label. During the development of the experiment, no rooting or organic matter was applied in the experimental area.
One day before the nematicide application, and then every 30 d for 24 months (total time of the experiment), root samples were collected in each repetition. Each sample consisted of the roots of three follower suckers between 1.5 and 2.5 m high from recently flowered plants or prompt to bearing. In front of each follower sucker, a hole of 26 cm long, 13 cm wide and 30 cm deep (soil volume of 10.14 L) was dug at the plant base using a shovel. All the roots found were collected and placed in labeled plastic bags and delivered to the INIAP (Instituto Nacional Autónomo de Investigaciones Agropecuarias) laboratory in coolers.
In the laboratory, the root samples were registered and processed as soon as possible, and when it was necessary, stored in a refrigerator (General Electric) at 6-8oC until being processed. The roots were rinsed to remove the soil, separated into living roots (white or cream-colored roots), dead roots by plant-parasitic nematodes (with symptoms of nematode damage, with necrosis, but without root decay), and dead roots by other causes (rotten roots by excess water, snapping). Then the roots were left to dry off the surface moisture and weighed (Cas computing scale precision 5 kg ± 1 g). During the root separation process, in some roots, it was necessary to cut some damaged parts, which were classified accordingly. The total root weight corresponds to the sum of living roots, dead roots by plant-parasitic nematodes, and dead roots by other causes.
The living and dead roots by nematodes were cut into 1-2 cm length pieces, and after homogenization, 10 g were randomly selected. These roots were macerated (Taylor and Loegering, 1953) in a kitchen blender (Osterizer; Sunbeam-Oster, USA) for two periods of 10 s at medium speed with a resting period of 4 s in between, and nematodes were recovered in a 0.038 mm (400 mesh) sieve. The nematodes were identified at the genus and species level when possible, based on the morphological characteristics under a light microscope, following the key of Siddiqi (2000). The population densities of all plant-parasitic root nematodes were recorded, and the values were converted to numbers per 100 g of roots.
At the beginning of the experiment, and 12 and 24 months after the first nematicide application, 90 randomly selected bunches of each treatment (15 per useful replicate) without plot edges, edge drains, cable edges or dompings were evaluated. Bunches were harvested by calibration starting when bunches reached 10 weeks of age. The bunch was harvested when in the second hand, the central fruit of the outer whorl had a diameter of at least a grade of 45 (35.5 mm-diameter). If in week 13 fruits did not reach the required minimum grade of 45, they were harvested with the grade they had. The harvest age, date of harvest, number of hands, applied dehanding, bunch weight (Tru-Test electronic scale XR3000 Kg ± 1g) and calibration of the central fruit of the outer whorl of the second hand were registered. To calculate the ratio, which is the number of boxes of 18.14 kg given by each bunch, a reduction of 20% was considered because it is the average of the farm, which includes 12% of rachis and 8% of non-marketable fruit. With the data of the number of bunches harvested in 2015 in the area where the experiment was located and the number of plants per hectare, the initial ratoon was estimated. In addition, with the age of bunches and harvest dates, the ratooning (number of bunches per production unit per year) was estimated at 12 and 24 months.
Root and nematode data were averaged by experimental plot across the 24 months, excluding the first evaluation pre-treatment application. The composition of the plant-parasitic nematode population was determined before treatment application, and then for the average of the 24 root samplings. Data of root weights before treatment application, and thereafter for the average of the 24 root samplings, were subjected to ANOVA by Proc GLM of SAS and mean separation by LSD-test. The number of nematodes was analyzed with generalized linear models, using the log transformation as link function and negative binomial distribution of the errors for the first nematode sampling alone, and thereafter for the average of the 24 nematode samplings together after the application. Bunch weight, number of hands per bunch, fruit calibration in the second hand, ratio, ratooning, and number of boxes of 18.14 kg per hectare per year (97% bunch recovery, 1,406 bunches x ratio x ratooning) were averaged for each repetition and harvest, and subjected to ANOVA and mean separation using LSD-test in PC-SAS® version 9.4.
Results
In the root sampling carried out before treatment application, no differences were found in the content of living roots (P=0.7148), dead roots by nematodes (P=0.2897), dead roots by other causes (P=0.4873), total roots (P=0.9799) and living root percentage (P=0.3373). The contents varied between 27.0 and 38.6 g for living roots; the dead roots by nematodes ranged from 3.9 to 10.4 g; the dead roots by other causes oscillated from 1.3 to 9.8 g, and the total roots from 40.3 to 45.8 g per follower sucker (Fig. 1A-D). The percentages of living roots in the sucker ranged between 66.0 and 87.8% (Fig. 1E). Similarly, in this sampling, no difference was detected among treatments in the populations of R. similis (P=0.8674), Helicotylenchus spp. (P=0.5294) and total nematodes (P=0.2458), which corresponds to the sum of the plant-parasitic nematode species detected (Fig. 2A-C). Nematode populations among treatments varied: R. similis between 7,267 and 21,200, Helicotylenchus spp. between 17,333 and 31,633, and total nematodes between 27,733 and 42,167 individuals per 100 g of roots. The composition of the nematode population before treatments application was: 31.5% of R. similis, 68.2% of Helicotylenchus spp. with a negligible amount of Meloidogyne spp. and Pratylenchus spp. (data not shown).

FIGURE 1 Fresh root weight (g) of living roots (A), dead roots by nematodes (B), dead roots by other causes (C), total roots (D), and percentage of living roots per sucker in banana (Musa AAA cv. Williams) plants treated with different number of nematicide cycles per year. Each point is the average of six repetitions. In each repetition, a hole of 26 cm long 13 cm wide, and 30 cm deep was dug at the base and in front of three follower suckers from 1.5 to 2.5 m high to collect all roots. V*= one nematicide cycle was with Verango®.
Root content and nematode populations through the 25 samplings are presented in Figures 1 and 2. Across the different samplings, root content and nematode populations followed a similar trend in all the treatments. After treatments application, when comparing the average of the 24 samplings (Fig. 3), differences were found among treatments in the contents of living roots (P=0.0003) and dead roots by nematodes (P=0.0009). The highest increase in living roots was observed in plants treated with three nematicide cycles per year with 21 and 17%, followed by the plants treated according to the economic nematode threshold of 2,500 plant-parasitic nematodes per 100 g of roots, which resulted in four applications per year with 16% (Fig. 3A) compared to untreated control. The application of nematicides reduced the dead of roots by nematodes between 20 and 46% (Fig. 3B). In agreement to the increase of roots in plants treated with nematicide and their lower dead of roots by nematodes, these plants had the highest (P<0.0001) percentage of living roots, which varied between 74.6 and 81.7%. These results contrast the 67.3% found in the untreated control plants (Fig. 3E). The grams of dead roots by other causes (P=0.0707) and total root (P=0.1570) weight were similar among treatments, ranging from 2.2 to 3.4 g, and from 41.2 to 44.9 g per sucker, respectively (Fig. 3C-D).
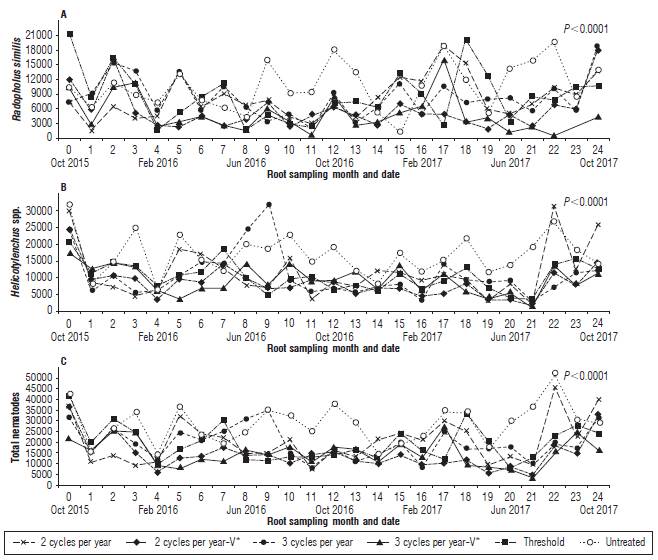
FIGURE 2 Number of Radopholus similis (A), Helicotylenchus spp. (B) and total nematodes (C) per 100 g of banana (Musa AAA cv. Williams) roots treated with a different number of nematicide cycles per year. Each point is the average of six repetitions. In each repetition, a 26 cm long 13 cm wide, and 30 cm deep hole was dug at the base and in front of three follower suckers from 1.5 to 2.5 m high to collect all roots. V*= one nematicide cycle was with Verango®.
The biggest nematode population per 100 g of roots of R. similis (P<0.0001), Helicotylenchus spp. (P<0.0001) and total nematodes (P<0.0001) was found in the untreated plants (Fig. 2 and Fig. 4). Compared to the untreated plants, nematicide treatments reduced R. similis between 20 and 49%, Helicotylenchus spp. between 31 and 50%, and the total nematode populations between 29 and 49% (Fig. 4A-C). Changes in the nematode population composition were observed by averaging the 24 samplings taken after treatment applications, with R. similis increasing to 45.8%, Helicotylenchus spp. decreasing to 52.2%, and Meloidogyne spp. and Pratylenchus spp. remaining negligible with 1.4% and 0.4%, respectively (data not shown).
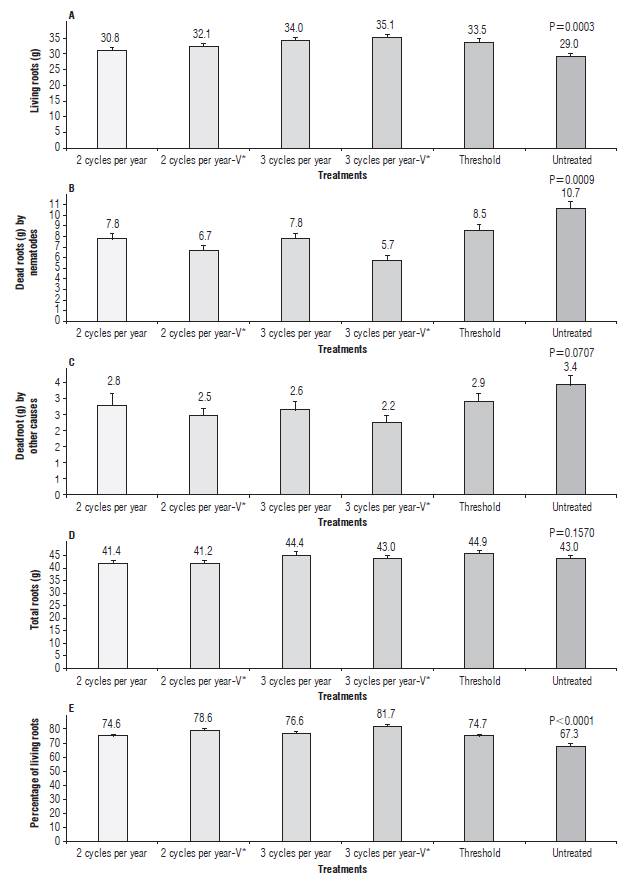
FIGURE 3 Average fresh root weight (g) of living roots (A), dead roots by nematodes (B), dead roots by other causes (C), total roots (D) and percentage of living roots (E) per follower sucker in banana plants (Musa AAA cv. Williams) treated with different number of nematicide cycles per year. Each bar is the average of 144 observations (24 samples per six repetitions), and in each repetition, the data are the average of three follower suckers. In front of each follower sucker, a hole of 26 cm long, 13 cm wide, and 30 cm deep was excavated at the base, and all roots were collected. V*= one nematicide cycle was with Verango®.
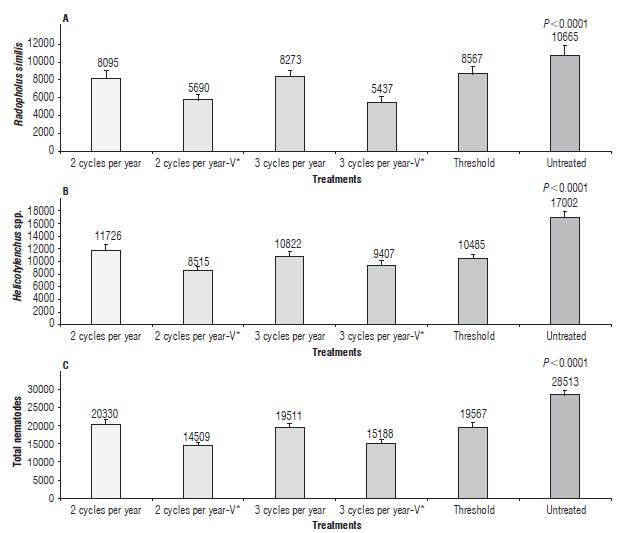
FIGURE 4 Number of Radopholus similis (A), Helicotylenchus spp. (B) and total nematodes (C) per 100 g of banana roots (Musa AAA cv. Williams) treated with a different number of nematicide cycles per year. Each bar is the mean ± standard error of 144 observations (24 samplings per six repetitions) and in each repetition, the data are the average of three follower suckers of 1.5-2.5 m high. A 26 cm long, 13 cm wide and 30 cm deep hole was dug in front of each follower sucker and all roots were collected. V*= one nematicide cycle was with Verango®.
The data of the three harvests carried out at the beginning of the experiment, and at 12 and 24 months after the first treatment applications are presented in Table 2. Bunch weight (P=0.1961) was similar among treatments at the initial harvest, varying between 34.8 and 37.9 kg per bunch (Tab. 2). In this initial harvest, the ratio (P=0.1926), which fluctuated between 1.53 and 1.67, and number of hands (P=0.4120), which varied between 8.9 and 9.3 hands per bunch, were also similar among treatments (Tab. 2). In congruence, the number of boxes per hectare (P=0.1922) which ranged from 3,600 to 3,923 (Tab. 2) and the calibration (P=0.1612) of the central fruit of the outer whorl of the second hand, which varied between 35.6 and 36.2 mm (data not shown), were similar among treatments. The initial ratooning in the experimental area was 1.67 bunches harvested in each banana stool per year (Tab. 2), which is equivalent to an interval between crop harvests of 218.5 d.
TABLE 2 Banana (Musa AAA cv. Williams) yield parameters according to the number of nematicide cycles per year and cost-benefit relation at the second and third harvest. Sell price of each box of 18.14 kg was US $6.15.
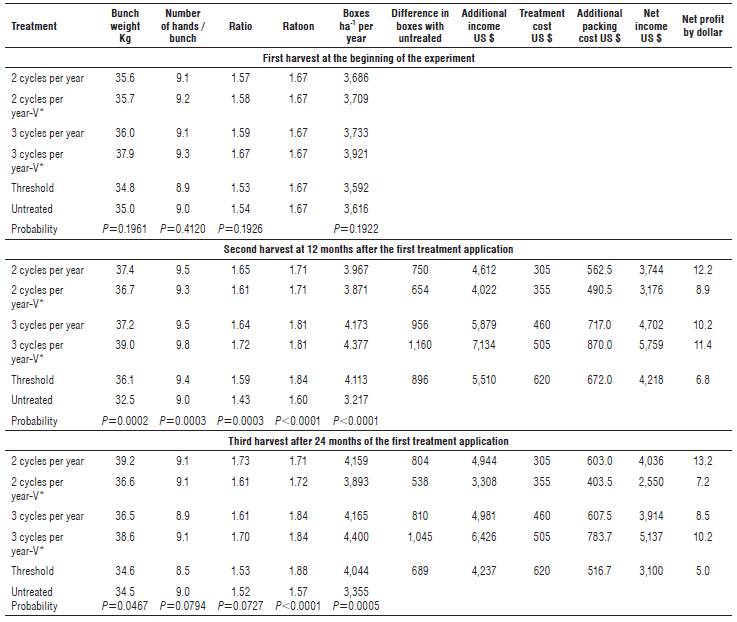
Ratio= number of boxes of 18.14 kg per bunch (80% of the bunch weight was packed (20% rejection that includes 12% bunch stalk and 8% rejected bananas) / 18.14 kg per box). 1,450 plants per hectare from which 97% of the bunches were processed (1,406 bunches), ratoon= number of bunches harvested per each banana stool by year, boxes per hectare per year= (1,406 bunches * ratio * ratoon). Each value is the mean of six replicates, and in each replicate, 15 bunches were harvested. V* one nematicide cycle was with Verango®. Net profit= additional income - treatment control cost - banana box packing cost of US $0.75 each. Counter® 15GR $150, Verango® $200, Vydate® 24SL $150, Rugby® 10G $160, and Mocap® 15GR $170 per hectare.
In the second harvest, carried out 12 months after the first treatment applications, an increase between 3.6 and 6.5 kg (11-20%) was observed in bunch weight (P=0.0002) except the bunches of the untreated plants (Tab. 2). In agreement to these results, treatments with nematicide increased the number of hands (P=0.0003) between 0.3 and 0.8 (3-10%), ratio (P=0.0003) between 0.16 and 0.28 (11-20%), ratoon-ing (P<0.0001) between 0.11 and 0.24 (7-15%) and number of boxes (P<0.0001) between 671 and 1,158 (20-36%) per hectare per year (Tab. 2). In comparison to the first harvest, which was performed at the time of establishing the experiment, treatments with nematicide increased the number of boxes between 190 and 510 (5-14%) per hectare per year, while in the untreated control a reduction of 408 (11%) boxes was found.
In the third harvest, 24 months after the first application of the treatments, differences in bunch weight (P=0.0467, Tab. 2) were found again. The maximum difference with the untreated plants was 4.7 kg (13.5%). The number of hands (P=0.0794) and ratio (P=0.0727) were similar among treatments, varying between 8.5 and 9.1 hands per bunch and between 1.53 and 1.73 boxes per bunch, respectively (Tab. 2). In all treatments with nematicide applications, an increase in ratooning (P<0.0001), varying between 0.14 and 0.31 (9-20%) was observed (Tab. 2). In parallel, in treatments with nematicide, an increase (P=0.0005) between 545 and 1,046 (16-31%) boxes per hectare per year was obtained compared to the untreated plants (Tab. 2).
The increases in bunch weight in the plants treated with nematicide led to an increase in the ratio (more boxes per bunch). In contrast, in the bunches of the untreated plants, a reduction of 0.11 units was observed in ratio at 12 months, which multiplied by the number of bunches per hectare per year gave a reduction of 246 boxes in the control. In the treatments with nematicide application, when the increase in their ratio in the second and third harvest was multiplied by the respective number of bunches harvested per hectare per year, an increase between 410 and 722 and between 22 and 497 boxes per hectare per year was found at 12 and 24 months, respectively (Tab. 2).
The increase in ratooning at 12 and 24 months after the treatments were applied was between 0.11 and 0.24 and between 0.14 and 0.31 units, compared to the control, respectively (Tab. 2). The initial ratooning of 1.67 from the experimental area was reduced in the untreated plants by 0.07 units at 12 months and by 0.03 additional units at 24 months (1.64 and 1.57, respectively) (Tab. 2). In contrast, in treatments with nematicide, the ratooning increased from 1.67 at the beginning of the experiment to 1.71-1.84, and from 1.71-1.88 at 12 and 24 months, respectively. This means that the interval between harvests at 12 and 24 months was reduced between 5.1 and 20.2 d and between 19.5 and 38.8 d in the nematicide treatments, while in the untreated plants, the interval was extended in 10 and in 4.7 additional d, going from 218.5 d at the beginning of the experiment to 228.6 and 232.7 d between harvests at 12 and 24 months, respectively. The planting density was 1,450 plants ha-1, of which 97% of the bunches (1,406) were processed. Then, when this number of harvested plants of 1,406 bunches per hectare was multiplied by the respective ratooning in the untreated plots, 2,245 and 2,205 bunches were harvested per hectare per year at 12 and 24 months, respectively. Compared to these untreated plants, in the nematicide treatments, between 159 and 342, and between 202 and 441 more bunches per hectare per year were harvested. This number ofbunches multiplied by the respective ratio resulted in values between 261 and 544 and between 348 and 676 more boxes per hectare per year at 12 and 24 months, respectively.
Discussion
In the sampling carried out before treatments application, no differences were found among treatments in root contents, populations of nematodes, or in the production variables evaluated at the time of establishing this experiment. This means that any difference that was found after applying the treatments was attributed to their effect. At the beginning of the experiment, the nematode population consisted mainly of Helicotylenchus spp. (68.2%) and R. similis (31.5%), reducing the proportion of Helicotylenchus spp. to 52.3% at the end of the experiment, while R. similis increased in the plant-parasitic nematode community to 45.8%.
This greater proportion of Helicotylenchus in banana nema-tode attacks has been observed in Cavendish plantations with insufficient control, as reported by Araya and Moens (2005) and Salguero et al. (2016). Helicotylenchus spp. is an ecto-endoparasite (Blake, 1966; Orion and Bar-Eyal, 1995; Gowen, 2000; Guzmán-Piedrahita, 2011a) that induces necrotic lesions on the surface of the roots. In contrast, R. similis is a migratory endoparasite that causes necrotic lesions along the entire root, in the epidermis, cortical parenchyma and vascular cylinder (Blake, 1966; Orton and Siddqi, 1973; Jackson et al., 2003; Guzmán-Piedrahita, 2011b). The high population of Helicotylenchus spp. and R. similis is favored because banana production is in perennial monoculture, although it is an annual crop.
The application of nematicide on the soil surface, in front of the follower suckers, reduced the populations of R. similis between 20 and 49%, of Helicotylenchus spp. between 31 and 50% and of total nematodes between 29 and 49% compared to the untreated control. This reduction in the population agrees with the results of Barriga et al. (1980) and Jaramillo and Quirós (1984), who found in average, a reduction between 49 and 82% of the plant-parasitic nematodes, respectively with different nematicide treatments. Araya and Cheves (1997a, 1997b) reported reductions of 22-63% for R. similis and 25-89% for Helicotylenchus spp. Quénéhervé et al. (1991a, 1991b, and 1991c) indicated reductions of 22.7-90.7% for R. similis and 32.5-100% for Helicotylenchus spp. In addition, Castillo et al. (2010) found drops of38-60% for Helicotylenchus spp., 24% for R. similis, and 25-33% for total nematodes. Moens et al. (2004) recorded reductions of 18-59% for the total plant-parasitic nematodes and Salguero et al. (2016) found decreases of 33-47% for R. similis, 36-65% for Helicotylenchus spp. and 35-59% for total nematodes. In agreement with the significant reduction of nematodes in treatments with nematicide, a significant increase in living roots of up to 81.7% and in the percentage of living roots of 21% were observed. Additionally, a decrease of up to 46% of dead roots by nematodes was recorded. Comparing treatments of 2 and 3 nematicide cycles per year (in which one of the cyles was with Verango®), those plants that with Verango® application showed a lower number of R. similis, Helicoty-lenchus spp. and total nematodes, and lower dead roots by nematodes and a higher percentage of living roots.
In response to the increased root health in plants treated with nematicide, an increase in bunch weight of 3.6-6.5 kg (11-20%) was found in the second harvest and up to 4.7 kg (13.5%) in the third harvest. The percentages of bunch weight increase recorded in this experiment were consistent with some of those cited by Vilardebó and Guerout (1976) between 12 and 123% and by Gowen (1993) between 16 and 45%. On the other hand, these percentages were lower than those reported by Araya and Cheves (1997a; 1997b) (22.1% and 40.8%, respectively), and than the ones found by Stanton and Pattison (2000) (44%), Moens et al. (2004) (45%), and Quénéhervé et al. (1991a) (48%).
The reductions in the interval between harvests are congruent with Quénéhervé et al. (1991b), who found a cumulative reduction in time to harvest according to the cycle of 28 d in the first, 57 d in the second and 128 d in the third harvest cycle in plants treated with nematicides. Similarly, Quénéhervé et al. (1991a) and Gowen (1995) report an increase in the harvest period from 13 to 32 and from 22 to 40 d, respectively, in plants infected with nematodes that were not treated compared to those with nematicide application. In congruence with this extension in the period to harvest, Roderick et al. (2012) reported an increase of 13.6 more days to harvest in Mbwazirume banana plants to which they added nematodes compared to plants without the addition of nematodes.
The highest number of boxes per hectare per year was due to the application of nematicide that resulted in a significant reduction of nematodes, which led to an increase in the percentage of living roots that favored water and nutrients uptake. This, in turn, allowed a better growth of the crop, which led to higher bunch weights, ratio, and ratoon. In the second and third harvest, nematicide treatments produced from 671 to 1,158 (12.2 to 21.0 t) and from 545 to 1,046 (9.9 to 19.0 t) more boxes of 18.14 kg per hectare per year than plants of the untreated plots, at 12 and 24 months of the treatment application, respectively. This means that nematode control increased production between 21 and 36% and between 16 and 31% at 12 and 24 months of treatment application, respectively. The smallest increases in production, 24 months after applying the treatments, probably indicate the proximity of the optimum yield; at that point, maintaining adequate control of the pest would stabilize the production, until the natural senescence of the crop begins.
The observed percentages of yield increase agreed with some of the percentages compiled by Gowen and Quénéhervé (1990), who mentioned increases of 14-263%, and Gowen (1995) who reported increases of 5-275%. However, these percentages were lower than those reported by Stan-ton and Pattisson (2000) of 46%. The increases in production found were in line with that reported by Cubillos et al. (1980), who cited increases of more than 300 boxes of 20.0 kg (6.0 mt), Quénéhervé et al. (1991b) who indicated increments in production of 523 to 1,157 boxes (9.5 to 21.0 mt), Pattison et al. (1999) who reported increases of 655 to 953 boxes of 13 kg (8.5 to 12.3 t), Araya and Lakhi (2004) who cited increments of 1,245 boxes of 18.14 kg (22.6 t), and Salguero et al. (2016) who found increases of 545 to 832 boxes of 18.14 kg (9.9 to 15.1 t) per hectare per year, controlling nematodes through the application of nematicides.
The highest yield (number of boxes per hectare per year) was observed in plants treated with three nematicide cycles per year. These results agree with that reported by Araya (2003), who registered higher yields as the number of ne-maticide cycles per year increased in Costa Rican banana plantations infected with nematodes. These increases in production as a result of nematodes control are in parallel with Guerout (1972), Charles et al. (1985), Quénéhervé et al. (1991a, 1991b), and Salguero et al. (2016), who cited negative and significant linear correlations between the populations of R. similis, Helicotylenchus spp. and total nematodes with bunch weight in bananas.
The high population of Helicotylenchus spp. and the increases in production achieved with the application of nematicides indicated that their parasitism reduces growth, development, and production. These results are in accordance with observations by McSorley and Parrado (1986), Gowen and Quénéhervé (1990), Chau et al. (1997), Barekye et al. (1998, 2000), Gowen (2000), Guzmán-Piedrahita (2011a), Coyne et al. (2013), and Salguero et al. (2016), who reported that H. multicinctus and H. dihystera damaged the banana root system and reduced yield between 19% (Speijer and Fogain, 1999) and 34% (Reddy, 1994). Additionally, Sijmons et al. (1994) indicated that the induction and maintenance of feeding sites of Helicotylenchus spp. causes physiological changes in the structure of cells. In the case of R. similis, it is well supported that it reduced the yield in banana (Gowen and Quénéhervé, 1990; Gowen, 1993, 1995; Araya, 1995, 2004; Guzmán-Piedrahita, 2011b; Roderick et al., 2012; Coyne et al., 2013).
The presence of nematodes with different parasitic habits, R. similis migratory endoparasite and Helicotylenchus spp. an ecto-endoparasite, most likely exacerbates root damage, since lesions can develop at feeding sites and through root tissue. In addition, plants often activate post-infection resistance mechanisms, even in cases where the population of nematodes increases over time and the nematode-plant interaction is compatible. Therefore, together these processes can represent high energy expenditure for plants which can interfere with the filling and development of the bunch. Given that both nematode genera cause damage to the crop, for the implementation of options for their management, the population of all present plant-parasitic nematodes should be considered, as has been suggested by Araya (2004), Ramclam and Araya (2006), Salguero et al. (2016), and Aguirre et al. (2016a, 2016b).
During the development of the experiment, the market price of a box of 18.14 kg of bananas was US $6.15, and of a nematicide application cycle including the application cost per hectare was US $150 for Counter® 15GR, US $200 for Verango®, US $150 for Vydate® 24SL, US $160 for Rugby® 10GR, and US $170 Mocap® 15GR. The costs of the fertilizer, control of black Sigatoka and weeds, plant propping, and other tasks were the same for the control plots and those treated with nematicide since the increase recorded was for the bunch weight, ratio and ratooning. The additional net income from the increase in yield deducted the cost of labor of US $0.75 for packing each additional box. The cost of the product and its application was from US $3,266 to $5,750 and from US $2,587 to US $5,144 per hectare per year at 12 and 24 months after treatments application, respectively. This net gain agrees with that indicated by Pattison et al. (1999) who reported amounts between US $2,494 to US $5,910 per hectare per year. This means, that for every dollar invested in nematode control, at 12 months, the net profit ranged from US $6.8 to $12.2, and at 24 months from $5.0 to $13.3. Despite the higher production in the plants that received three nematicide cycles per year, in which one of the cycles was with Verango®, the highest net profit was obtained with two nematicide cycles per year, with a return of US $12.2 and US $13.3 at 12 and 24 months, respectively, of applied treatments for every dollar invested in nematode control.














