Introduction
The export of avocado fruit (Persea americana Mill) generates important commodities to several countries such as Mexico, Indonesia, The United States of America, Dominican Republic, Colombia, Chile, and Peru (FAO, 2018). Avocado crops exhibit several limiting factors when producing high quality fruit to satisfy a steady growing demand from international markets. In Colombia, a fast growth in the planted area has been observed in recent years leading to an increase on the economic profit at the agricultural sector. However, consistent issues derived from the lack of technical and scientific knowledge by some farmers related to the correct implementation of agronomical practices have been identified, along with the increase in the planted area. These problems include planting in flat areas susceptible to flooding and, hence, to diseases, bad phytosanitary quality of seedlings, lack of knowledge about environment, temporal and spatial factors involved in disease development, little training for correct identification and management of diseases and disorders, and shortage of certified laboratories to perform diagnostics (Tamayo, 2007; Ramírez-Gil et al., 2014; Ramírez-Gil et al., 2017; Ramírez-Gil, 2018).
Diseases are considered as important challenges to crop management because if not detected accurately and treated promptly, they may cause large economic losses depending on the cultivar and edapho-climatic conditions where the crop is grown. In addition, phytosanitary measures implemented for disease control are accompanied by adverse effects to human health and the environment (Zentmyer, 1980; Menge and Ploetz, 2003; Tamayo, 2007; Ramírez-Gil et al., 2014, 2017).
Root rot caused by Phytophthora cinnamomi Rands is considered the most important disease of avocado plantations in the world. Root rot is highly influenced by the abiotic factor hypoxia-anoxia caused by poor soil aeration, and usually by rain flooding (Stolzy et al., 1967; Zentmyer, 1984; Ramírez-Gil et al., 2014, 2017; Hardham and Blackman, 2018). In addition, other microorganisms, such as Phytophthora heveae Thompson., Phytophthora parasitica Dastur., Verticillium sp. Nees, Armillaria mellea (Vahl) P. Kumm., Cylindrocladium sp. Morgan, Rosellinia sp. De Not, Fusarium solani (Mart) Sacc., Fusarium oxysporum Schlecht., Fusarium sp. Link ex Grey, Rhizoctonia sp. DC, Phymatotrichum omnivurum (Duggar) Hennebert., Cylindrocladiella sp. Boesew, Cylindrocarpon sp. Wollenw, Pythium sp. Pringsheim, Gliocladiopsis sp., and the nematodes Helicotylenchus sp. Steiner, Rotylenchulus sp. Linford and Oliveira, and Pratylenchus sp. Filipjev, have been reported as causal agents of avocado root rot. However, their frequency and importance depend on many factors associated with the environmental and edaphic conditions of the planted areas and the agronomical management of the productive system (Tamayo, 2007; Vitale et al., 2012; Ramírez-Gil and Morales-Osorio, 2013; Ramírez-Gil et al., 2014, 2017; Parkinson et al., 2017).
After root rot, the most frequently identified pathologies in avocado crops are scab (Elsinoeperseae, (Jenkins) Rossman & W.C. Allen, synonymy = Sphaceloma perseae, Jenkins), anthracnose of fruits and dieback of buds and branches (Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. Teleomorph = Glomerella cingulata (Stoneman) Spauld. & H. Schrenk), leaf and fruit spot (Pseudocercospora purpurea (Cooke) Deighton. = Cercospora purpurea), fruit rot (Rhizopus stolonifer Ehrenb. Fr Vuill. and Dothiorella sp.), graft rot (Lasiodiplodia theobromae Pat. Griffon & Maubl. = Botryodiplodia theobromae), algal leaf spot (Cephaleuros virescens Kunze), and sooty mold (Capnodium sp., and Asteridiella perseae F. Stevens Hansf.) (Zentmyer, 1984; Menge and Ploetz, 2003; Tamayo, 2007; APS, 2017; Sharma et al., 2017; Giblin et al., 2018).
In Colombia and other tropical countries, information about diagnosis of pathologies and disorders is scarce; therefore, an accurate and prompt disease identification system is not fully implemented in all growing areas. Avocado diseases have become complex to identify and difficult to manage, not only because of the economic losses they generate, but also because of the symptoms caused by the different pathogenic agents (both biotic and abiotic) that can be easily confused at plant diagnostic laboratories by technical assistants and farmers (Ramírez-Gil et al., 2017; Tamayo, 2007; Ramírez-Gil, 2018). Incorrect diagnostics usually lead to inappropriate management practices; for example, most root rot diseased trees are diagnosed as infected by P. cinnamomi and controlled accordingly. However, such management practices can be effective if that is truly the causal agent; otherwise, they not only fail to solve the problem, but the sanitary conditions of the crop worsen resulting in a rapid decay and death of trees (Ramírez-Gil et al., 2017; Ramírez-Gil, 2018).
Accurate and prompt identification of the causal agent of a disease is the first and more important step for an appropriate management program. Thus, effective control measures can be established optimizing resources and reducing negative effects to human health and the natural environment (Agrios, 2005). Nowadays, different techniques and aspects should be included to implement a correct process of diagnosis of plant diseases and disorders, such as field symptomatology, pathogen isolation, macroscopic and microscopic microbial morphology, pathogenicity tests, Koch postulates, and molecular sequencing; this combination of methods is named polyphasic diagnosis (Taylor et al., 2000; Alvarez, 2004).
The objective of this research was to establish a polyphasic approach for the identification of avocado diseases caused by biotic causal agents or abiotic disorders under standard growing conditions of avocado cv. Hass crops in Antioquia, Colombia.
Materials and methods
Localization and sample processing
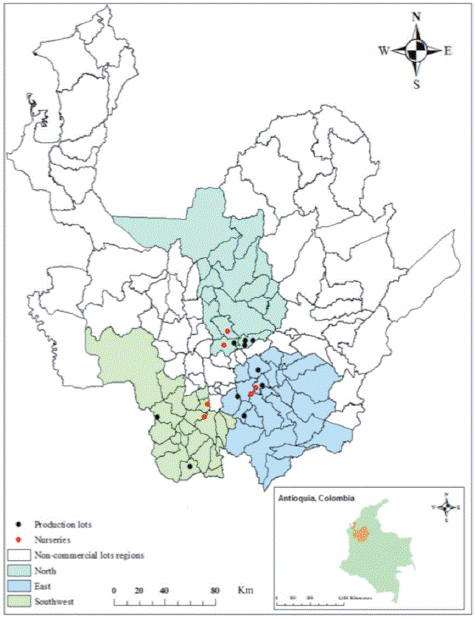
Ten commercial plots and six nurseries for production of avocado cv. Hass seedlings, located in the North, East and Southwest regions of Antioquia, Colombia, were surveyed during eight years for disease identification (2009-2016). Plot and nursery locations and their edaphoclimatic conditions are reported in Supplementary material 1 and 2. Plants showing disease symptoms were recorded and photographed, and tissue samples were collected and processed for causal agent or disorder identification as described below. Each avocado plot was managed according to local agronomical practices without further technical intervention. Presence of symptoms was evaluated in nurseries in seeds, roots, stems, and foliage; in crop plots, symptoms were evaluated in roots, stems, foliage, flowers, and fruits. Sample processing and pathogenicity tests were performed at the Laboratorio de Fitotecnia Tropical at Universidad Nacional de Colombia, Medellin campus.
Polyphasic diagnosis of avocado cv. Hass diseases and disorders
Symptom description
Plants showing any disease symptoms in nurseries and in crop fields were photographed and registered for eight years. Each symptom associated with a particular disease was described during the complete duration of the pathology or disorder.
Isolation and morphological characterization of microorganisms associated with symptomatic plants
Samples were collected from each plant showing visible symptoms potentially associated with pathogens. Afterwards, samples were covered with paper towels, placed in zip-pack plastic bags in Styrofoam containers and transported to the laboratory for further analyses. Once in the laboratory, samples were rinsed with tap water and non-ionic detergent for 1 min (30% of tween 20 in sterile distilled water (SDW)) and dried in paper towels at room temperature (22-25°C). A portion of sample tissues was incubated in humid chambers at 20°C and >90% relative humidity to corroborate if the microorganism found in the diseased tissue corresponded to the isolated on culture media. The remaining portion of the sample was sectioned in pieces (~1 cm, depending on the tissue) in sterile laminar flow cabinet and surface-disinfested in 70% ethanol for 30 s followed by rinsing in SDW for 30 s. Samples were then submerged in sodium hypochlorite (3% in SDW) for 30 s and rinsed in SDW for 30 s. Finally, samples were dried out on sterile paper towels and placed on semi-selective media culture.
For Phytophthora spp. vegetable juice V8-agar (V8-AACB) (V8-180 ml L-1 and 24 g L-1 of agar Difco, USA) amended with ampicillin (200 µg L-1), chloramphenicol (20 µg L-1) and benomyl (100 µg L-1) was used. For fungi, PDA acidified with lactic acid (PDA-A) (Difco, USA) and vegetable juice V8-agar (V8-AS) (Difco, USA) amended with streptomycin (100 µg L-1) were used. For bacteria, nutrient agar (NAB) (Difco, USA) supplemented with benomyl (50 µg L-1) and yeast-dextrose-calcium carbonate (YDCB) (Difco, USA), supplemented with benomyl (50 µg L-1) were used (Shaad et al., 2001). All media plates with samples were incubated at 25°C for 15 d under a photoperiod of 12 h of light and 12 h of darkness.
Colony growth habit, consistency, and color were registered for each isolate obtained. Micro-mounting of mycelia and spores was performed from purified isolates and plant tissues incubated in the humid chamber and observed under light microscopy coupled with differential interference contrast (DIC) (Nikon Eclipse E200). Species identification was performed following the taxonomic keys and guidelines by Barnett and Hunter (1972) and Seifert et al. (2011) for fungi; Erwin and Ribeiro (1996) for Phytophthora spp., and Mai and Mullin (1996) for phytoparasitic nematodes. For bacteria, standard biochemical tests and guidelines were followed as proposed by Shaad et al. (2001). For nematode extraction, soil samples were passed through a series of sieves of 250, 53 and 38 µm. Soil suspension collected in the last sieve was centrifuged for 3 min at 3800 rpm and re-suspended in a solution of SDW and sucrose (50%) and centrifuged again as described. The obtained supernatant was passed through the 38 sieve (Jenkins, 1964). In addition, root samples obtained from trees in-field conditions were washed in SDW and air-dried; then fine sectioning and histological mountings were performed for microscope observation based on the standard method used at the laboratory of Fitotecnia Tropical.
Biotic causal agents
In order to identify if the microorganism isolated from diseased tissues was associated with the symptoms observed under field conditions, pathogenicity tests were performed on avocado plants to fulfill the Koch postulates. Seeds from avocado fruits of cv. Hass of similar size and high phytosanitary conditions (based on visual inspection of absence of symptoms) were collected from plots planted with avocado (Supplementary material 1). Under laboratory conditions, seeds were surface-disinfected in sodium hypochlorite solution (3% in SDW), and then rinsed in SDW. In addition, pre-germination treatments were applied (seeds were cut in the upper, lateral and basal sides). Finally, seeds were then sown in previously autoclaved quartz sand (0.1 MPa and 121°C, per two cycles of 1 h each) (Ramírez-Gil et al., 2014).
Plants were maintained under net-house conditions at an average relative humidity of 90% and a temperature between 18 and 24°C. When plants had five fully expanded leaves and well-developed secondary root system, isolated microorganisms were inoculated on the same organs of avocado plants from which they were initially isolated (i.e. roots, stems, leaves). Pathogenicity tests of isolates associated with flowers and fruits were carried out on these same tissues collected from healthy plants in commercial plots, which were inoculated and incubated in humid chambers at 20°C and >90% relative humidity based on the standard method used at the laboratorio de Fitotecnia Tropical.
In order to process microorganisms isolated from roots, 200 ml of inoculum solution (PDA-SDW) at a concentration of 1*103 - 1*106 infective propagules ml-1 were inoculated following the method reported by Ramírez-Gil et al. (2014). A similar solution (200 ml of inoculum at 1*103 infective propagules ml-1) plus 2 g l-1 of agar (Difco, USA) for adhesion improvement was prepared for inoculation of microorganisms isolated from stems, foliage, fruits, and flowers, and was further sprayed over the surface of the corresponding tissue.
Inoculum of microorganisms that were not possible to isolate in the used media culture (i.e. Capnodium sp. and Cephaleuros virescens) was obtained by washing and scrapping infected tissues with SDW and a scalpel. Inoculum suspension was adjusted to 1 * 10-6 infective units ml-1 and inoculated as previously described.
Causal agents of biotic disorders
Experiments were conducted to reproduce symptoms induced by each potential abiotic factor of disorder observed under field conditions. Scion-rootstock compatibility in cv. Hass grafting was tested. Scions from avocado cv. Hass trees were grafted onto plants grown from seeds from the same cv. Hass rootstock and on plants grown from seeds of West Indian race rootstock collected from three avocado genotypes selected in San Pedro de Uraba, Antioquia, Colombia (9°46'49.6" N, 75°16'52.3" E, 239 m a.s.l). For hypoxia-anoxia conditions in roots, the guidelines reported by Ramírez-Gil et al. (2014) were followed. Root atrophy was tested by monitoring avocado seedlings growth in small plastic bags (5 cm height and 3 cm of diameter) for more than eight months. In addition, root shape and horizontal and vertical lengths were measured. Herbicide toxicity was reproduced by spraying foliage and fruits with N-(phosphonomethyl) glycine (glyphosate) in SDW at doses used by farmers. Applications were performed with a manual agricultural sprayer (0.03 MPa) coupled with hollow cone nozzle to produce a droplet size of 50 of volume mean diameter (VMD). Sunburn damage was tested by exposing fruits selected on the filling stage to direct sunlight during two hours per day for a week. Hail damage on fruits was simulated by impacting frozen balls of water (3.65 g of weight and 1 cm of radius on average) on fruit epidermis, launched from one meter of distance, with a terminal velocity of 14.15 m s-1 and impact energy of 4.29 Jules using a toy gun.
All pathogenicity tests were performed for a time period of 90 d, except fruits that were carried out only for 30 d. The variable measured was the incubation period (Ramírez-Gil et al., 2014). In pathogenicity tests, microorganisms were re-isolated in the same media culture as described. Pathogenicity tests were carried out under net-house conditions at a temperature of 18-22°C, 75-95% relative humidity and a photosynthetically active radiation (PAR) of 650-1920 µmol photons m2 s-1.
Molecular identification of pathogenic microorganisms
All microorganisms that were positive in the pathogenicity tests were further analyzed by sequencing the genomic ITS region as a complement of morphological identification. DNA was purified from mycelia using the DNeasy Plant Mini (QIAGEN®) following the manufacturer's instructions. DNA quality and quantity were measured using a Nanodrop spectrophotometer and separation by agarose gel electrophoresis (1%) in TBE 1X at 90 v stained with EZvision following the manufacturer's instructions and visualized and photographed under UV light in a trans-illuminator (Biometra, Göttingen, Germany).
Purified DNA was used as template for amplification of the genomic ITS region by the polymerase chain reaction (PCR) in a Thermal cycler (T3 Biometra, Göttingen, Germany), using amplification conditions and primer2 combinations ITS'4-ITS'5 (ITS 4: 5'- GGA AGT AAA AGT CGT AAC AAG G -3'; ITS 5: 5'- TCC TCC GCT TAT TGA TAT GC -3') and ITS'1-ITS'4 (ITS 1: 5'- CTTGGT CAT TTA GAG GAA GTA A-3'; ITS 4: 5'- GGA AGT AAA AGT CGT AAC AAG G -3') reported by White et al. (1990). Amplified DNA fragments were separated and visualized by agarose gel electrophoresis (1.5%) with 2µl of SYBR green (10 mg ml-1) following the manufacturer's recommendations and the size of the amplified products was determined by comparison to a Generuler 100 pb DNA ladder (Fermentas). PCR products were further purified with the QIAquick PCR Purification Kit (QIAGEN®) following the manufacturer's instructions.
Purified PCR products were sent for sequencing to Macrogen (Republic of Korea) following the company's guidelines (http://foreign.macrogen.com/eng/business/ngs_overview.html). Sequences were manually cleaned and edited using the software Bioedit 6.0.6 and Chromas 1.45. Then, sequences were aligned using Clustal W algorithm implemented in Bioedit software. Sequence identity was obtained by comparison with sequences in databases available at NCBI using the algorithm Blast implemented in the web page (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi).
Results and discussion
Description of causal agents and disorders associated with root rot and stem rot
Biotic causal agents
Phytophthora cinnamomi Rands
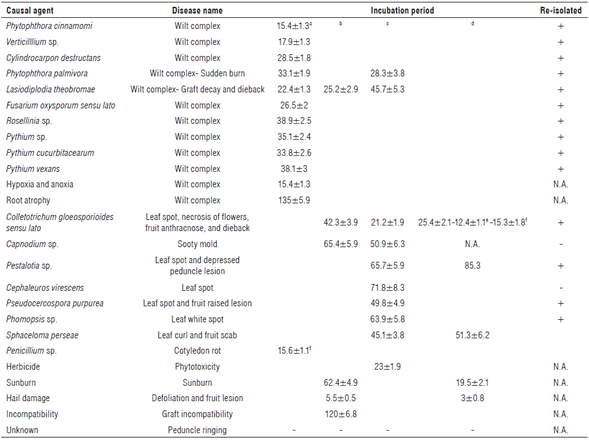
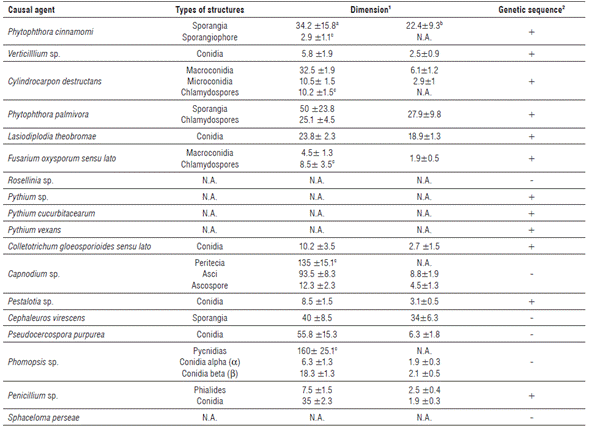
This oomycete was identified in all stages of plant growth and development under field conditions. Symptoms associated with P. cinnamomi were foliar yellowing, tissue flaccidity, growth retardation, excessive flowering and fructification in adult trees, total defoliation, dieback, secondary and tertiary root rot, and plant death. Isolates showed rosette growth on PDA and cottony and white mycelia. Under light microscopy, mycelia were coralloid and with rounded nodules; sporangia were non-papillated, ellipsoid and no caduceus; chlamydospores were absent and there was presence of non-branched sporangiophores (Tab. 1 and Fig. 1 A-F). Genomic sequences showed the highest percentage of homology with those registered as P. cinnamomi in the NCBI database. Morphology and genomic sequences corresponded to P. cinnamomi (Erwin and Ribeiro, 1996; Ramírez-Gil et al., 2014) (Tab. 1). The incubation period was 20 d (Tab. 2).
TABLE 1 Morphological structures and DNA ITS sequences of microorganisms associated with avocado cv. Hass diseases.
aLength; bwidth; cfor these structures, the diameter was the mean of 20 measures. N.A.: not apply. 1 data in ,µm observed under 10 and 40 X (see Fig. 1 and 3). 2 +: sequence was obtained; -: sequence was not obtained respectively.
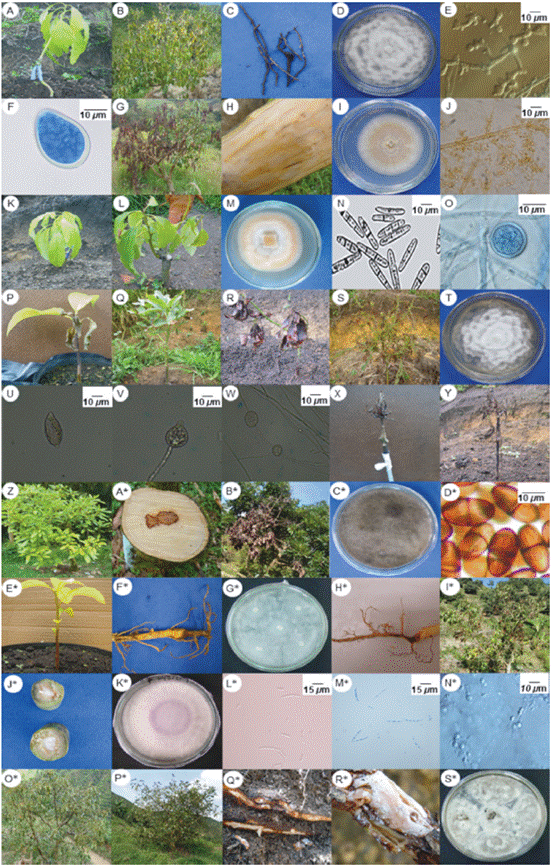
FIGURE 1 Symptomatology and structures of microorganisms associated with biotic causal agents of pre-harvest diseases in avocado cv. Hass. A to F: Phytophthora cinnamomi. G to J: Verticillium sp. K to O: Cylindrocarpon sp. P to Y: Phytophthora palmivora. Z to D*: Lasiodiplodia theobromae. E* to G*: Pythium sp. H* to N: Fusarium oxysporum sensu lato. O* to S*: Rosellinia sp.
Verticillium sp. Nees
A fungus was isolated from plants showing wilt, foliar yellowing, and stunted growth. Through time, these avocado trees exhibited hemilateral branch death and total death in seedlings. As a typical feature, infected leaves kept adhered to the plant. Transversal cut in the stems showed brown coloration in the vascular bundles. Rot was observed in part of the primary, secondary and tertiary roots. Isolates showed hyaline, floccose and septate mycelia. Under light microscopy, branched verticilate conidophores were observed, with unicellular hyaline and ovoid conidia (Tab. 1 and Fig. 1 G-J). All characteristics along with genomic sequences corresponded to Verticillium sp. and specific species Verticillium albo-altrum and Verticillium dahliae (Barnett and Hunter, 1972; Seifert et al., 2011; Ramírez-Gil et al., 2014). The incubation period was about 17.9 d (Tabs. 1 and 2).
Cylindrocarpon sp. Wollenweber
A fungus was isolated from seedlings at the nursery stage or recently transferred to field conditions. Plants exhibited generalized yellowing, stunted growth, flaccidity, defoliation and dieback symptoms. In media culture, velvety aerial mycelium with color variation from beige to creamy brown was observed. Under light microscopy, plain macroconidia, straight or curved, cylindrical-fusoid in shape, with a maximum of three septa were observed. Few microconidia were observed. They were plain, ellipsoid, cylindrical, straight or curved, with or without septa and scarce chlamydospores (Tab. 1 and Fig. 1 K-P). Morphology and genomic sequences corresponded to Cylindrocarpon destructans and Cylindrocarpon sp. (Barnett and Hunter, 1972; Seifert et al., 2011; Ramírez-Gil et al., 2014) (Tab. 1). The incubation period was 28.5 d (Tab. 2).
Phytophthora palmivora Butler
Most isolates of P. palmivora were obtained from roots and from the base of the stem from seedlings at the nursery stage. They were also obtained from plants recently transferred to field conditions and in a lower percentage from adult avocado trees. Foliar yellowing, stunted growth, cankers at the base of the stem, and root rot were symptoms associated to P. palmivora. Isolates in PDA exhibited stellate, hyaline and cottony mycelia, with variable sporangia of ovoid, ellipsoid, obpyriform, spherical, cenocytic and prominent papillae, with spherical chlamydospores present (Tab. 1 and Fig. 2 Q-X). Morphology and molecular sequences coincided with P. palmivora (Erwin and Ribeiro, 1996). The incubation period was 33.1 d (Tab. 2).
Lasiodiplodia theobromae (Pat.) Griffon & Maubl.
Generalized yellowing, stunted growth, foliar yellowing and pith rot characterized avocado trees infected with this microorganism. Grayish fast-growing colonies becoming dark-black through time, without reproductive structures were observed in PDA media. When mycelia were placed on avocado fruits and stems, hyaline and pigmented conidia was observed, with or without septa, with spherical or ellipsoid shape, thick cell wall and septate paraphyses (Tab. 1 and Fig. 1 Y-E*). Morphology and genomic sequences indicated that isolates were L. theobromae (Barnett and Hunter, 1972; Seifert et al., 2011). The incubation period measured in the pathogenicity tests corresponded to 22.4 d. (Tab. 2).
Pythium sp. Pringsheim
Oomycete isolates were obtained from seedlings at the nursery stage and plants recently transferred to field conditions. In these plants, foliar yellowing, stunted growth and generalized wilt was observed, including small cankers in the base of the stem and pervasive root rot. Isolates exhibited abundant aerial mycelia in PDA media, with cottony texture, white and extended or rosette shape. Under light microscopy, coenocytic hyphae with thickenings were observed (Tab. 1 and Fig. 1 F*-H*). The characteristic described and genomic sequences allowed the identification of two different and one non-identified species (Erwin and Ribeiro, 1996) named Pythium cucurbitacearum, Phytopythium vexans and Pythium sp. (Tab. 1). The three oomycete species reproduced similar symptomatology with incubation periods between 35.4 and 38.1 d (Tab. 2).
Fusarium oxysporum sensu lato Schlect. emend. Snyd. & Hans.
This fungus was isolated from avocado plants in all growth and development stages under field conditions. In seedlings, F. oxysporum caused root rot and damping off; under field conditions it induced pervasive wilting in leaves and fruits, which remained adhered to the plant. Cross sections of the stem revealed necrosis in the vascular bundles. Fast growing colonies in PDA were observed, with cottony texture and variable color. Hyaline, almost-straight or semi-curved macroconidia with three to five septa were observed under light microscopy along with abundant microconidia with oval or ellipsoid shape and hyaline and spherical chlamydospores (Tab. 1 and Fig. 1 I*-P*). All characteristics coincided with Fusarium oxysporum sensu lato (Barnett and Hunter, 1972; Fourie et al., 2011; Seifert et al., 2011). Phytopathological tests showed an incubation period of 26.5 d (Tab. 2).
Rosellinia sp. (Fr.) De Not.
This fungus was isolated from plants in all stages of development showing general wilt and mild yellowing. Internal necrosis and superficial cracks accompanied by an abundant mass of white mycelia were identified in the root system of affected plants. Under light microscopy, septate and branched mycelia of brown color forming an abundant reddish stroma were visualized (Tab. 1 and Fig. 1 Q-V). All results pointed to Rosellinia sp. as the fungal species involved in diseased tissues (Barnett and Hunter, 1972; Seifert et al., 2011; Ramírez-Gil et al., 2014). The incubation period measured during pathogenicity tests was 38.9 d (Tab. 2).
Root atrophy
Symptoms, such as foliar yellowing, stunting, and sparse foliage, were identified in avocado plants at nursery and field stages of development. In these plants, root atrophy was observed as a short, thick and forked main root, with reduced presence of secondary or tertiary roots. No microorganisms were isolated from plants affected with this pathology. Under laboratory conditions, symptoms were reproduced by sowing plants in small bags (25 cm of diameter and 36 cm of height). Reproduction of symptoms took 135 d (Tab. 2 and Fig. 2 A-E).
Hypoxia-anoxia
Leaf flaccidity, mild yellowing, necrosis of the leaf apex, stunted growth, total defoliation and death at the final stage depicted this pathology that was identified in all stages of plant development. Root rot was observed in main and secondary root systems, which was characterized by blue-greyish coloration visualized at dissection. No microorganisms associated to this pathology were isolated and symptoms were reproduced using plants grown in autoclaved soils with high humidity. Reproduction of symptoms took 15.4 d (Tab 2 and Fig. 2 F-H). Symptomatology corresponded to disorders caused by low oxygen levels in the soil known as hypoxia-anoxia (Stolzy et al., 1967; Ramírez-Gil et al., 2014, 2017).
Biotic causal agents of diseases and disorders affecting stems, leaves, flowers, fruits and seeds on avocado cv. Hass
Sudden leaf burn caused by Phytophthora palmivora
This pathology was observed mainly on plants at the nursery stage or recently transferred to field conditions. Plants exhibited leaves with burned edges of dark brown color leading to wrinkling toward the adaxial side of the leaf blade.
In advanced stages of infection, necrosis and defoliation were observed. Isolated and identified microorganisms and genomic sequences corresponded to P. palmivora (Tab. 1 and Fig. 1 R to X). The incubation period in pathogenicity tests was 28.3 d. (Tab. 2).
Graft rot and dieback caused by Lasiodiplodia theobromae
Stem rot in the grafting zone between the scion-rootstock was observed on plants at the nursery stage or recently transferred to field conditions. Symptoms advanced rapidly leading to defoliation and dieback. In adult trees, this microorganism caused dieback. Leaves became brown and remained adhered and the stem showed necrosis with deep black color. L. theobromae was consistently isolated from these diseased tissues and it was confirmed by genomic sequences as previously described (Tab. 1 and Fig. 1 Y, Z, C* and E*). Symptoms were reproduced in the grafting zone and leaf apex in seedlings, with incubation periods of 25.2 and 45.7 d, respectively (Tab. 2).
Seed rot, foliar spot, flower necrosis, anthracnose in fruits and dieback caused by Colletotrichum gloeosporioides sensu lato
The pathology caused by this fungus was identified during all stages of plant development. Symptomatology included necrosis in plumule and radicle, stem rot in the grafting zone between the scion-rootstock, tiny black foliar spots with necrotic center, branch dieback, and tissue rot from the apical zone to the base of the stem. Flowers showed black necrosis. Mummification was observed during all developmental stages in fruits that remained adhered to the plant. In developed fruits, small black spots on the epidermis were visualized, and a pink mass was observed under high humidity.
Colonies in PDA with a highly variable in color, white, greyish or salmon, of fast growth with spongy and dense mycelia were obtained from the different affected tissues. Conidia were hyaline, unicellular, cylindrical, oval, and ellipsoid-fusiform with one side tapered and the other rounded (Tab. 1 and Fig. 3 A to I). Morphology and genomic sequences corresponded to Colletotrichum sp. (Barnett and Hunter, 1972; Seifert et al., 2011; Sharma et al., 2017; Giblin et al., 2018). This group has been recently referred to as a species complex known as Colletotrichum gloeosporioides sensu lato, and their accurate identification at the species level requires the use of other methods beyond the scope of the present research (Weir et al., 2012; Sharma et al., 2017; Giblin et al., 2018). The incubation periods of pathogenicity tests carried out in leaves, stems, flowers, and fruits were 21.2, 42.3, 25.4 and 12.4 d, respectively (Tab. 2).

FIGURE 3 Symptomatology and structures of microorganisms associated to causal agents of pre-harvest diseases on avocado cv. Hass. A to I: Colletotrichum gloeosporioides sensu lato. J to M: Capnodium sp. N to R: Pestalotia sp. S to W: Phomosis sp. (teleomorph Botryosphaeria sp.). X and Y: Cephaleuros virescens. Z* to D*: Pseudocercospora purpurea. E* to H*: Sphaceloma perseae. I* to L*: Penicillium sp.
Sooty mold by Capnodium sp.
Symptomatology was characterized by a black layer of mycelia on the adaxial or upper side of the leaf blade, mainly observed in adult trees and in a lower percentage in nursery seedlings. Mycelia were easily removable from affected leaves, stems, and fruits. In advanced stages it caused leaf fall. It was not possible to isolate any microorganism in media culture. Under light microscopy, the structures observed from diseased leaves revealed dark-brown mycelia with short septa, globose perithecia, almost pedunculate, bitunicated ascus and ascospores (Tab. 1 and Fig. 3 J-M). Characteristics corresponded to Capnodium sp. (Barnett and Hunter, 1972; Seifert et al., 2011). An incubation period of 50.9 d was measured in the pathogenicity tests (Tab. 2).
Foliar spots and depressed lesion on the peduncle caused by Pestalotia sp.
This disease was identified affecting leaves and fruit peduncle in all stages of plant development. In leaves, a brown irregular spot of variable size preferably located in the leaf apex was observed. In the fruit peduncle, this pathogen caused a black depressed lesion with black dots over it, which corresponded to pathogen structures in advanced stages. In PDA, a fungus of white color, cottony texture, and with deep black oily protrusions was isolated. Under light microscopy, abundant fusoid conidia with 2-3 septa, of brown color in the center but hyaline with appendices in the apices were observed (Tab. 1 and Fig. 3 O-S). The associated microorganism was identified as Pestalotia sp. (Barnett and Hunter, 1972; Seifert et al., 2011). The incubation periods during pathogenicity tests in leaves and fruit peduncles were 65.7 and 85.3 d, respectively (Tab. 2).
White spot by Phomopsis sp.
This disease was only identified in leaves of adult trees, characterized by small spots (<2mm) with necrotic borders and white-greyish center, which in advanced stages coalesced to generate large necrotic areas. From diseased tissues, a fast-growing microorganism was isolated in PDA, of white color evolving to grey, with unilocular and globular pycnidia, inside of which hyaline, unicellular a conidia constricted in the center with blunt base were observed. Longer, filiform, flexuous or curved β conidia without septa were also observed (Tab. 1 and Fig. 3 I-X). Characteristics observed corresponded to Phomopsis sp. (Barnett and Hunter, 1972; Seifert et al., 2011). The incubation period in the pathogenicity tests was 63.9 d (Tab. 2).
Foliar spot caused by Cephaleuros virescens
This pathology was observed in all stages of plant development causing green, yellow or orange, rounded and raised spots of velvety appearance on the leaf blade surface. No microorganisms were isolated from lesions with media used in the present work. Under light microscopy, coenocytic mycelia with sporangiophores showing oval sporangia were observed (Tab. 1 and Fig. 3 Y-Z). Data obtained corresponded to C. virescens (Barnett and Hunter, 1972). Symptoms were reproduced after an incubation period of 71.8 d in the pathogenicity tests (Tab. 2).
Foliar spot and raised lesion in avocado fruits by Pseudocercospora purpurea
This pathology preferably appeared in adult trees affecting leaves and fruits but it did not occur commonly in the present study. Small foliar spots (<5 mm), brown in color, showing necrotic margins and irregular shape were observed. In fruits, superficial cracks in the epidermis that evolved to raised lesions were identified. In PDA, isolates exhibited slow growth, cottony colonies that were white at the beginning but became gray and dark through time. Under light microscopy, basal stroma, from which straight or slightly curved conidiophores emerged, was observed. Conidia were cylindrical in shape, elongated with several septa (Tab. 1 and Fig. 3 A*-E*). Characteristics indicated that isolates corresponded to P. purpurea (Barnett and Hunter, 1972; Seifert et al., 2011). An incubation period of 49.8 d was measured in the pathogenicity tests (Tab. 2).
Leaf curl and fruit scab by Sphaceloma perseae
Although rarely seen during the present study, this pathology presented affected leaves and fruits inducing small spots (<10 mm) of irregular shape and light brown color that occasionally induced leaf curling. In fruits, irregular, corking, depressed or raised lesions were observed. A microorganism from white to grey color was isolated in PDA which showed cylindrical or elliptical, lunate or falcate conidiophores of variable size (Tab. 1 and Fig. 3 F*-I*). S. perseae was identified from findings reproducing symptoms in the pathogenicity tests (Barnett and Hunter, 1972; Seifert et al., 2011) (Tab. 2).
Cotyledon rot by Penicillium sp.
Brown irregular lesions that grew covering most of the cotyledon surface were observed. In PDA, a fast-growing fungus was isolated, with light yellow color that became dark through time, cottony texture with abundant conidia, hyaline and septate hyphae, with simple or branched conidiophores, 3-7 phialides and globular conidia in short or divergent chains (Tab. 1 and Fig. 3 J*-O). Morphological characteristics and DNA sequences coincided with Penicillium sp. (Barnett and Hunter, 1972; Seifert et al., 2011). Pathogenicity tests were positive for the isolated microorganism (Tab. 2).
In this study, no pathogenic nematodes or bacteria were found based on the methodology used (Mai and Mullin, 1996; Shaad et al., 2001).
Abiotic causal agents of diseases and disorders affecting stems, leaves, flowers, fruits and seeds on avocado cv. Hass
Herbicide phytotoxicity on leaves and fruits
Leaves affected by herbicides became light brown and detached; total defoliation and plant death may be observed when large areas were exposed. Spots were usually scattered in an irregular manner through foliage. In fruits, herbicides produced irregular black spots that remained unaltered for long periods. No microorganisms were isolated from those lesions; symptoms were reproduced at 13 d after plants were sprayed with herbicide (Tab. 2 and Fig. 2 I to K).
Fruit and stem sunburn
Fruits showed mild yellowing with reddish hues or a rounded lesion of dark brown color when exposed to direct sunlight because of partial defoliation or large fruiting. In stems, green-yellow coloration that became brown or black was identified when directly exposed to strong sunlight. No microorganisms were found associated to this disorder and the pathogenicity tests reproduced symptoms when tissues were continuously exposed to direct sunlight. Reproduction of symptoms was observed at 19.5 and 62.4 d for fruits and stems, respectively, after sun exposure (Tab. 2 and Fig. 2 L-O).
Rootstock-scion incompatibility
This abnormality was observed in all stages of plant development from nursery to adult trees. The disorder was characterized by thickening in the grafting zone between the rootstock and the scion. More frequently, scion showed a bigger diameter in the stem than the rootstock, causing stunted growth and sometimes plant death. Symptoms of stunted development were reproduced in plants when compared to compatible grafts (Tab. 2 and Fig. 2 P-Q).
Defoliation and lesions caused by hailstorm
Hail caused defoliation to a degree that depends on the hailstorm intensity, but that may lead to total defoliation. On fruits, hail caused deep (5 mm) and small rounded lesions (<4 mm) that became necrotic spots through time. Over the lesions, a corking tissue developed that remained until harvest causing damage to fruit quality. The worst damage was caused in the first stages of fruit development. The reproduction of symptoms was of 3 days in the performed pathogenicity tests (Tab. 2 and Fig. 2 R-S).
Peduncle ringing
This disorder was more frequently observed on small fruits (<10 mm in diameter) and during the development stage known as fruit filling. It was expressed as a ring in the fruit peduncle, which acquired round shape and purple hue from the peduncle to the apex, which may remain adhered to the plant showing progressive dehydration until mummified or detached. No microorganisms were isolated from diseased plants and it was not possible to replicate the symptoms in the pathogenicity tests; therefore, this disease remains of unknown etiology (Fig. 2 T).
Avocado root rot was associated with ten biotic and two abiotic causal agents inducing similar symptoms. As this pathology has multi-agent etiology, accurate and prompt diagnosis is difficult and frequently leads to the application of wrong control methods that may cause large losses (Zentmyer, 1984; Machado et al., 2008; Vitale et al., 2012; Ramírez-Gil et al., 2014, 2017; Parkinson et al., 2017). Phytophthora palmivora, L. theobromae, Fusarium oxysporum sensu lato, Phytopythium vexans, Pythium cucurbitacearum, and Pythium sp. were identified as associated with root rot causal agents in our research; in addition, they have not been previously reported in Colombia (Tamayo, 2007; Ramírez-Gil et al., 2014, 2017). Pythium cucurbitacearum had not been reported before in the avocado crop, being this the first report worldwide (APS, 2017). Similarly, the abiotic factor root atrophy had not been previously reported as a root rot causal agent in Colombia and around the word (Tamayo, 2007; Ramírez-Gil et al., 2014, 2017).
Colletotrichum gloeosporioides sensu lato and L. theobromae excelled as pathogens associated to stems, leaves, fruits and seeds, and P. palmivora was mainly involved in diseases affecting roots, the base of the stem and leaves. As these pathogens may affect several avocado tissues, their importance should not be neglected in an integrated disease management program. Disorders of abiotic origin associated with symptoms in stems, leaves, flowers and fruits identified in the present work have been reported before with slight variation in symptom expression. This may be due to the local edaphoclimatic conditions in the regions studied which may influence symptomatology (Zentmyer, 1984; Menge and Ploetz, 2003; Sanders and Korsten, 2003; Tamayo, 2007; Machado et al., 2008; APS, 2017). Diseases produced by Phomopsis sp., hail damage and phytotoxicity by herbicide had not been reported before for Colombia.
Despite the fact that avocado diseases have been studied from a long time, some pathologies remain of unknown etiology such as the peduncle ringing. Potential causal agents have been proposed including humidity deficit during fruit filling, warm and dry winds, high nitrogen and potassium or low magnesium levels (Toerien, 1979) and secondary microorganisms. Rootstock-scion incompatibility has been attributed to phylogenetically distant genotypes; however, it has been also observed in closely related genotypes of Persea sp. This problem may cause large losses in advanced stages of plant development when trees are in full production. Further research is needed to clearly elucidate the main factors predisposing to this disorder and how to prevent them (Frolich et al., 1958).
Correct and prompt diagnosis of avocado diseases is of paramount importance for successful crop production. Polyphasic approaches for disease identification have proven to be useful for appropriate integrated crop management because misleading identification of problems may cause large losses and plant death. Disorders caused by abiotic factors are usually accompanied by secondary microbial colonization mainly due to dead tissue. This fact may induce a wrong causal agent identification and incorrect control measures, leading to the aforementioned economic and plant losses. As many diseases were identified in the present research, permanent monitoring and epidemiological studies should be implemented to promptly identify and control new or expanding pathologies in tropical environments where avocado cv. Hass has been growing in Colombia. Nationwide studies should also be considered to detect prevalent or emerging diseases at local areas different from the ones studied here.
This work is a comprehensive contribution for the detection and diagnosis of the most frequent pathologies of avocado production systems under the evaluated conditions. Here, we display a multistep approach of disease identification including morphological characterization of associated microorganisms, detailed characterization of the symptoms associated with each of the pathologies and disorders and different factors that may be involved in disorders that affect this crop. The present research may be a guide to be consulted by avocado producers, technical assistants, professors, students, government agencies and other actors involved in the avocado industry. On the other hand, it is necessary to continue with studies to look for alternative technologies for the early, sensitive and accurate detection and diagnosis of diseases to maintain and increase sustain-ability for this crop.
Conclusion
The polyphasic approach developed in this work for diagnosis of cv. Hass avocado pre-harvest pathologies and disorders was an alternative that allowed the appropriate identification of microorganisms and causal agents of the most frequent diseases and disorders in roots, stems, leaves, flowers, and fruits of avocado crops in tropical conditions in Antioquia, Colombia. Additionally, our research may be used as a guide for the detection of different types of phytosanitary problems in avocado.