Introduction
Cereals including barley (Hordeum vulgare L.) are the most widely consumed food group in the world, particularly in developing countries, due to their low cost and high energy content (Lopez et al., 2008). Indigenous groups of the inter Andean region of Ecuador have cultivated barley as far back as the third quarter of the sixteenth century. Barley was quickly accepted among the indigenous people of the Sierra and, although they had very good native crops, the barley grain was planted for use as food and for brewing (Patino, 1963). Most of the barley grain is used for human consumption, including traditional machica (toasted and ground barley flour) to make coladas (beverages) and pinol (a traditional hot drink of Ecuador made from a mixture ofmachica, panela and some sweet species), flour and baked bread, and as grain for preparing soups and desserts. In addition, barley is used in the malting industry for brewing and to a lesser extent as fodder for cattle (Villacres, 2008).Barley is an important source of vitamins, minerals, proteins, antioxidants and dietary fiber (Baik and Ullrich, 2008; Havrlentová et al., 2011). Dietary fibers in cereals are complex carbohydrates with ten or more monomeric units. These fibers are cellulose, hemicellulose, pectin, and β-glucans, among other compounds, and their structural differences determine their physical, chemical and physiological properties (FAO, 2010). β-glucans constitute approximately 75% of the cell wall of the endosperm of the barley grain (Sarwar et al., 2013). The structure of β-glucan has a significant impact on human health, specifically its effect of lowering total cholesterol and reducing the risk of cardiovascular diseases (Havrlentová et al., 2011). β-glucan has been shown to reduce postprandial glucose by increasing the viscosity in the upper digestive tract and providing a feeling of satiety sooner. A balanced diet rich in dietary fiber provides immense benefits to cardiovascular health, reducing between 10-20% the low-density lipoproteins (LDL). In addition, the rheological properties of β-glucans have promoted dietary fiber incorporation into different food with the aim of improving the stability, texture and shelf life of food products (Wood, 2007; Alminger and Eklund-Jonsson, 2008; Regand et al., 2011; Pizarro et al., 2014). The objectives of this study were to measure the β-glucan content of accessions in the seeds of Ecuadorian elite barley collection of the Instituto Nacional de Investigaciones Agropecuarias (INIAP), guide the use of accessions, establish the relationship among β-glucan, viscosity and dietary fiber and determine the effect of several industrial processes on β-glucan content.
Materials and methods
Plant material and raw sample processing
This research was conducted at the Santa Catalina Research Station in the laboratories of the Nutrition and Quality Department of INIAP, Ecuador. Initial characterizations were based on the β-glucan content of seventy barley accessions from elite lines and varieties of yield trials grown in 2017 at Santa Catalina Research Station. A complete block design was applied with three replicates. One sample of each repetition was taken for the analysis (Supplementary material 1) for a total of 70 materials. The grains were ground in an analytical mill (Retsch Zn 200®, Hann, Germany) with a 42-mesh sieve (355 μm) to obtain a homogeneous powder. To express the results on a dry basis, the moisture content of the flour was determined by weight difference drying.
β-glucan analysis
The Megazyme kit (Megazyme International, Ireland) was used specifically for the mixed-linkage (1→3) (1→4)- β-D-glucan assay. This enzyme assay allows for the direct analysis of β-glucan in raw and cooked flour slurries in which the fine particles are suspended and hydrated in a buffer solution at pH 6.5 (Doehlert et al., 1997). The suspension was incubated with a lichenase enzyme to depolymerize into tri-, tetra-, and higher degree oligosaccharides, which were then hydrolyzed to glucose using β-D-glucosidase. The D-glucose produced was quantified using the reagent glucose/oxidase/peroxidase (Mcclear and Glennie-Holmes, 1985). Three replicates from each sample were analyzed in a completely randomized design (CRD) using ANOVA with the statistical software InfoStat (Di Rienzo et al., 2015). The LSD (0.05) test was used to perform means separation.
Viscosity determination
Viscosity was determined using a Cannon Frenskey glass capillary viscometer following the method by Alvarado and Aguilera (2001).
Soluble dietary fiber quantification
The soluble dietary fiber was quantified using a modified Method 991.42 and Method 993.19 of AOAC (AOAC, 1995). The modification consisted in the dispersion of the sample in a pH 7.4 phosphate buffer and the addition of bile and pancreatic enzymes. An ANOVA was conducted to analyze the data which was organized under a completely randomized design (CRD) using InfoStat (Di Rienzo et al., 2015). An LSD test (0.05) was applied to conduct means separation. Twelve accessions with a β-glucan content greater than 2.48 were selected for further assays to determine viscosity, soluble dietary fiber, and the effect of four industrial processes on β-glucan content.
Effect of processing on β-glucan content of grain
Twelve barley accessions with high β-glucan content in the initial tests were further analyzed to determine the effect on the β-glucan content of four different industrial food processes listed here: scarification, roasting, cooking, and malting. One kilogram of grain was scarified in a Strong-Scott 07810 abrasive system (Strong-Scott, Minnesota, USA) for 1 min. A second set of grains (1 kg) was roasted in a Barber Colman, 471 P model oven (Rockford, Illinois, USA) at 120°C for 10 min at a speed of 10 rpm. A third set of grains (1 kg) was cooked in an open pot using an electric stovetop at 91°C for 40 min, maintaining a grain:water ratio of 1:3. At the end of the set time, the cooking water was removed and the grain was dried in an oven (Memmert, Schwabach, Germany) at 60oC for 5 h. A fourth set of grains (1 kg) was malted, a process which includes soaking the grains in water at 16°C for 12 h, then draining the water and allowing the grains to germinate in a Barber-Colman oven (Rockford, Illinois, USA) at 90% humidity at 16°C for 72 h. At this point in the malting process, the grains were dried in a Barber-Colman dryer to stop the germination process. After processing, the grains were milled and analyzed for β-glucan content, with the results expressed as a percentage of sample dry weight. There were three replicates per treatment, and the data were analyzed in a completely randomized design (CRD) with factorial structure (AxB) using InfoStat (Di Rienzo et al., 2015). An LSD (0.05) test was conducted for the means separation of significant factors.
Results and discussion
β-glucan content in seventy accessions of raw barley
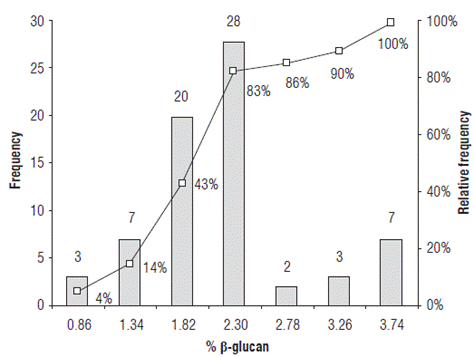
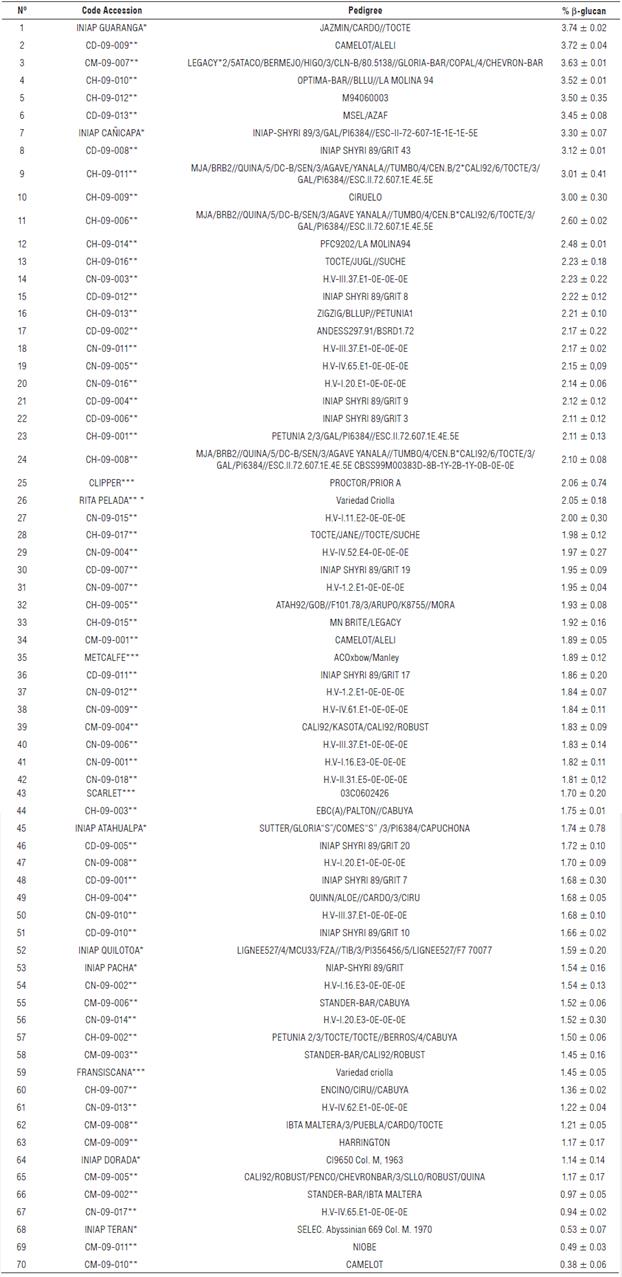
Barley accessions showed significant variability in β-glucan content with mean values from 0.38% to 3.74% (w/w) (Supplementary material 1). Twenty-eight of the accessions had a β-glucan content which ranged from 1.82% to 2.30% (w/w) and 86% of the evaluated accessions had β-glucan content below 2.78% (Fig. 1). The accessions with a β-glucan content lower than 2.10% (w/w) have the potential to be used in the brewing industry and in the animal feed industry for monogastric animals. In studies with chickens and rats, the main deleterious component of barley grain is the hydrocolloidal β-D-glucan. Probably because of the increase in the viscosity of the intestinal contents this causes poor performance for chicks (Griffey et al., 2010). β-glucans decreases the activity of endogenous enzymes, alters intestinal morphology and modifies microflora composition and activity, reducing nutrient digestion and absorption and increasing the susceptibility to disease (Jacob and Pescatore, 2014). The β-glucan contents of the remaining 32 cultivars and accessions were higher than 2.10% (w/w). Those in the range of 2% - 4% (w/w) can be used for animal feed, while those with β-glucan content higher than 3% can be used for human consumption, either consumed directly as cooked or roasted grain or processed as ingredients for different food products. Barley accessions with high β-glucan content can also be used as raw material for obtaining soluble dietary fiber (SDF) to enrich different foods. Barley bran, which is rich in β-glucan, can be incorporated into oriental noodles and pasta products. Consumption of cereal fiber, such as that from barley, has been shown to have an effect on cardiovascular health by reducing low-density lipoprotein (LDL) cholesterol by about 10-20%. In addition, cereal fiber helps to reduce blood glucose levels and provides a greater sense of satiety (Wood, 2007).
Based on the low β-glucan content, the following varieties are used as checks for malted barley accessions: Metcalfe, Scarlett, Clipper and Harrington. According to this,accessions with β-glucan content of 2.10% (w/w) or lower are appropriate for the brewing industry, since high levels of β-glucan in a malt means incomplete cell wall degradation, diminished mobilization of starch and proteins for hydrolysis, and lower malt extract values, hence, less available nutrients for fermentation during brewing. Moreover, β-glucans, in association with polyphenols, proteins and other polysaccharides, can be components of chill hazes, which form during beer storage and give the first visual impression to the consumer of beer quality (Jadhav et al., 1998). The amount and molecular weight of β-glucans in malt affects the viscosity of must and beer. Barley β-glucans also negatively influence turbidity in beer (Speers et al.,2005).
There were no significant differences when hulled barley accessions were compared to hulless barley accessions for β-glucan content. No statistical differences were found for β-glucan content in the 27 two-row accessions with relation to the 43 six-row accessions with mean values of 2.0% and 1.9% (w/w), respectively. The barley accessions with the highest β-glucan content were INIAP-Guaranga and barley breeding lines CD-09-009 and CM-09-007 with β-glucan contents of 3.74% (w/w), 3.72% (w/w) and 3.63% (w/w), respectively.
β-glucan, viscosity and soluble dietary fiber in selected barley cultivars
The twelve accessions with the highest β-glucan content were selected for further assays to determine viscosity, soluble dietary fiber, and the effect of four industrial processes on β-glucan content.
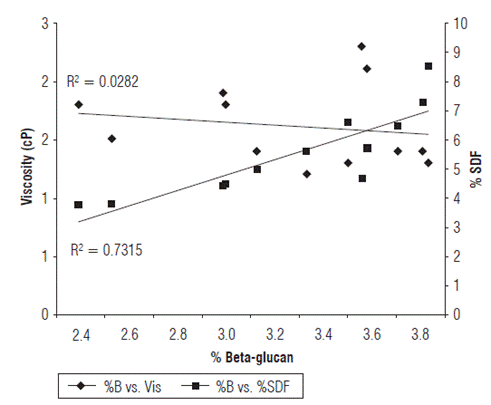
The correlation coefficient showed a low dependence of the β-glucan content on viscosity (Fig. 2), suggesting that in addition to β-glucan, viscosity is also affected by other components. These results are similar to those reported by Knutsen and Holtekj0len (2007), who analyzed 16 barley cultivars from Norway, Canada and Denmark and did not find significant differences in the β-glucan content. Their results confirm that β -glucan is located mainly in the al-eurone layer surrounding the endosperm, rather than in the grain husk (Knutsen and Holtekjølen, 2007). β-glucan content is influenced by genotypic and environmental factors (Doehlert et al., 1997; Jadhav et al., 1998), though the genetic background of barley is considered more important than environmental conditions as a determinant of the final β-glucan content of the grain (Doehlert et al., 1997). Both hulled and hulless (naked) barley have a higher percentage of Fe, P, Zn and K in their grains compared to the other cereals commonly grown in Ecuador. However, the mineral concentration in hulled barley decreases by approximately 32% after the pearling process (Villacrés and Rivadeneira, 2005), suggesting that an emphasis on breeding hulless or naked types will be beneficial to the overall nutrition of people who consume barley.
The correlation coefficient demonstrated a lack of a positive relationship between the β-glucan content and viscosity. Barley accessions with β-glucan content higher than 2.10% showed a high degree of correlation with soluble dietary fiber (SDF) concentration, suggesting that in addition to β-glucan, viscosity is also affected by other components. These can include arabinoxylans or pentosans which are not hydrolysed by β-glucanases (Fig. 2). Other authors indicate that water extracts of ground barley contain gums with low-molecular weight (about 200,000 D) β-D-glucans and pentosans (Prentice, 2000). Several studies indicate that the molecular structure and structural organization of β-glucan and arabinoxylan in oats and barley are important in determining their physical properties such as water solubility, viscosity (gelation) and digestibility (Lazaridou and Biliaderis, 2007; Izydorczyk et al., 2008; Skendi and Biliaderis, 2016). This correspondence is due to the fact that β-glucans are the major components of the SDF in barley (Yoon et al., 1995; Bunzel et al., 2001; Mudgil and Barak, 2013).
Effect of processing on β-glucan content of grain
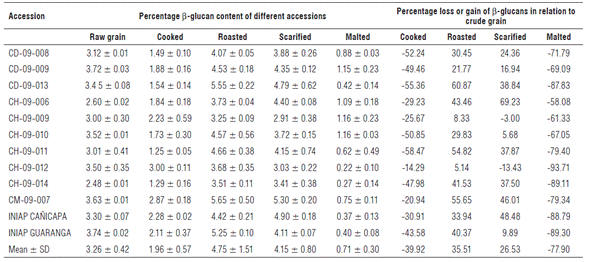
The aqueous cooking of barley grains caused a decrease of 39.9% in the β -glucan content of the 12 accessions evaluated compared to the β-glucan content of raw grain (Tab. 1). The accessions that had the greatest reduction compared to the raw grain were CH-09-011, CD-09-013, and CD-09-008, with a decrease of 58.5, 55.4 and 52.2%, respectively (Fig. 2). This could be attributed to the solubility of β-glucan in hot water. In contrast, the grain roasting process increased β-glucan content by 35.5% compared to the raw grain (Tab. 1). The accessions that showed a significant increase in β-glucan content with the roasting process were CD-09-013, CM-09-007 and CH-09-011, with a 60.9, 55.6 and 54.8% increase compared to the raw grain (Fig. 2). Roasting involves heat and mechanical shearing which may have affected the molecular and structural characteristics of β-glucans and their interaction with other barley components, thus having an impact on β-glucan content (Lazaridou and Biliaderis, 2007). The high temperatures during the roasting process may have also inhibited the activity of β-glucanases, so that β-glucan was not hydrolyzed and was present in higher concentrations (Lazaridou and Biliaderis, 2007).
In the scarified or polished grain, the β-glucan content increased by 26.5% compared to the raw grain. Three accessions (CH-09-006, INIAP-CAÑICAPA and CM-09-007) showed a significant increase in β-glucan content in relation to the raw grain, with increases of 69.23, 48.49, and 46.01% (Fig. 2). This effect could be due to the removal of the external peel adhering to the pericarp while retaining the outer cell layer where β-glucans are concentrated. Regarding this, Knutsen and Holtekjølen (2007) and Marconi et al. (2000) suggest that β-glucan content is not significantly affected by the polishing and scarifying process, though, in general, there could be an increase in the β-glucan content. Additionally, abrasion removes the seed coat, the aleurone and subaleurone layers, and the germ, resulting in polished grain rich in starch, β-glucans and protein (Jadhav et al., 1998).
The malting process decreased the β-glucan content by as much as 78.3% compared to the raw grain. The accessions that showed a greater reduction in β-glucan content due to the malting process were CH-09-012, INIAP-GUAR-ANGA, and CH-09-014, with a decrease of 93.71, 89.30 and 89.11% (Tab. 1) (Fig. 2), which can be attributed to the enzymatic activity during grain germination. The degradation of endosperm cell walls and the subsequent change in β-glucan levels during malting are largely related to the activity of β-glucanases, which depolymerize β-glucans and influence their reduction during the malting process (Zhang et al., 2002). Therefore, the content of β-glucan in the malted grain results from the initial content of each accession and the effect of the β-glucanases. However, the two characteristics have independent genetic effects.
Conclusions
An analysis of β-glucan in 70 barley accessions showed variations between 0.38% and 3.74% (w/w). Twelve of these materials had a β-glucan content greater than 2.10% (w/w) and were subjected to four industrial processes to determine their effect on the β-glucan content. This compound was increased in the grain by roasting and scarification, while cooking and malting caused a decrease in β-glucan content, helping to guide the use of the grain. Thus, accessions with a content higher than 3% can be used for human consumption, either consumed directly as cooked or roasted grain, or processed as ingredients for various food products. Accessions with a content of less than 2.10% have the potential to be used in the brewing and animal feed for monogastric animal industries.