Introduction
The mite Tetranychus urticae Koch is one of the most limiting arthropods for papaya cultivation in various producing regions of the world, including the province of Valle del Cauca in Colombia (Santa-Cecilia and Reis, 1986; Vieira et al., 2004; Moraes and Flechtmann, 2008; Gómez et al., 2014; Migeon and Dorkeld, 2006-2017). Tetranychus urticae feeds on a wide range of host plants (1140 plant species) that comprise diverse families (Migeon and Dorkeld, 2006-2017). It frequently begins colonization of host plants on the mature leaves, and as its populations increase, it invades the younger leaves (Marin et al., 1995; Seeman and Beard, 2011; Vásquez et al., 2012). The mite develops its colonies on the underside of leaves and has a CW type of life (complex and complicated spider web production) according to the classification of Saito (2010). Their web provides shelter and protection for the immature stages against predator attacks, toxic substances, and adverse climatic conditions (Moraes and Flechtmann, 2008; Badii et al., 2010). Two-spotted spider mites feed more commonly on older papaya leaves, which initially turn yellow on the upper sides and silver on the lower sides, followed by the appearance of necrotic areas and eventually leaf drop (Collier et al., 2007). This damage, predominating in the dry season, directly influences photosynthesis and increases exposure to the sun, with negative consequences for fruit production and marketability. For this reason, pesticide applications in papaya orchards occur year-round, with the well-known risks to the environment and human health (Marín et al., 1995).
Biological control of T. urticae has proven to be successful in different crops, and the predator mite Neoseiulus californicus (McGregor) is one of the major biological control agents of T. urticae in rose (Rosa spp.) crops in greenhouses of several countries (Marafeli et al., 2011). In Colombia, the use of predators of the genera Phytoseilus spp. and Amblyseius spp. has been reported as an effective control of the mite T. urticae in roses (Valcárcel-Calderón, 2013) as well as the effective use of the entomopathogenic fungus Isaria fumosorosea Wize (Castro, 2010). For this same species of Tetranychidae, the releases of phytoseids Phytoseiulus persimilis Athias-Henriot and N. californicus were very effective in nursery conditions for clementine (Citrus clementina Hort. ex Tan.) orchards in Spain (Sá, 2012). Alternative products based on the neem Azadirachta indica (Juss) have also successfully exercised control of T. urticae populations in strawberry plants (Soto et al., 2011). However, insecticides and acaricides have always played the central role in controlling this species of mite in different crops such as the papaya in Valle del Cauca (Gómez et al., 2014).
Currently, a large number of compounds with different chemical structures and application modes is used (Knowles, 1997; Dekeyser, 2005; Van Leeuwen et al., 2009). However, T. urticae has a high capacity for rapidly developing resistance to chemical products (Knowles, 1997, Van Leeuwen et al., 2008). Its high reproductive potential, inbreeding, the condition of producing haploid males and its short life cycle resulting in many generations per year, facilitate the rapid development of resistance to many acaricides after a limited number of applications (Nauen et al., 2000; Van Leeuwen et al., 2010). Among all the arthropods, T. urticae has the highest prevalence of resistance to pesticides (Van Leeuwen et al., 2010; Grbic et al., 2011). This very rapid resistance to new and commercially available chemical products affects integrated management programs (Cho et al., 1995). Additionally, the excessive use of these chemicals has increased the health risks of farmers and consumers, in addition to the negative impact on the environment (Morera, 2015).
Some of the most effective biological controllers for the control of T. urticae are Phytoseiidae mites such as P. persimilis and N. californicus (Cloyd et al., 2006; Rhodes et al., 2006). Both species possess important attributes such as a shorter development cycle than that of T. urticae, a rapid development of populations, and high search capacity and voracity, among other details (Acosta, 2000; Gómez et al., 2014). McMurtry et al. (2013) classify P. persimilis as a specialized predator of mite species of the genus Tetranychus (Type: Ia), and they classify N. californicus as a selective predator of mites of the family Tetranychidae (Type: II), which might be used in programs of integrated pest management.
Some studies have been conducted with Phytoseiidae mites in papaya but under controlled conditions. Collier et al. (2004) report Neoseiulus idaeus Denmark & Muma, 1973 in Brazil as a good candidate for biological control of T. urticae, based on laboratory results due to its high occurrence in papaya production systems with intensive use of acaricides, together with its favorable biological characteristics reported in the literature. López (2014) found that the predatory mites N. californicus and Amblyseius swirskii Athias-Henriot, 1962 are viable for the control of T. merganser in papaya cultivation under greenhouse conditions in Brazil.
Chrysoperla carnea (Neuroptera: Chrysopidae) is indicated as an important natural enemy of soft-bodied phytophagous arthropods, such as red spiders, in part because of its high voracity as well as its polyphagous nature (Pappas et al., 2011). It is a species with wide geographical distribution, tolerance to insecticides such as abamectins, and ease of breeding in the laboratory, which allows its mass production in different laboratories of Valle del Cauca (Nasreen et al., 2005; Cerna et al., 2012). Hassanpour et al. (2009) recommend including C. carnea in red spider management programs due to its good predatory potential.
Predatory mites of the family Phytoseiidae and predatory insects such as C. carnea are widely included in biological control programs in various crops, due to their ability to survive, reproduce and tolerate some chemical compounds (Cloyd et al., 2006). But a control exercised by these predators has not been evaluated on T. urticae in crops of C. papaya in Colombia. The hybrid Tainung-1 is the most cultivated material in the province with yield losses caused by T. urticae, considered to be the most limiting pest for the cultivation of papaya in this region of the country (Gómez et al., 2014). That is the basis for this research, aimed at evaluating the control exercised by P. persimilis, N. californicus and C. carnea on populations of T. urticae in papaya Tainung-1 in Valle del Cauca, under field conditions.
Materials and methods
Obtaining P. persimilis and N. californicus
Individuals of P. persimilis and N. californicus used for field trials were obtained from the commercial company Bichopolis-BioBee© SAS., located in the municipality of Tabio, Cundinamarca. The mites were packed and sent in 60 ml plastic bottles whose lid had a dispenser. Each bottle contained 1,000 predatory mites mixed with vermiculite. Shipments from Tabio were made by land following the route Tabio-Cali-Roldanillo (to the farm "La Pola") with an average time of 48 to 72 h from packing to release. The bottles contained the two species of Phytoseiidae, P. persimilis and N. californicus, in a 600:400 ratio, respectively, for each bottle.
Obtaining C. carnea
The individuals of C. carnea were obtained from the commercial company Perkins Ltda., located in the city of Palmira. Each package contained 100 eggs of C. carnea mixed with rice husk and Sitotroga cerealella eggs (Olivier) (Lepidoptera: Gelechiidae). These sealed packages were transported in a cooler with ice at the bottom, one day before the hatching of the eggs, from Palmira to the farm "La Pola" (municipality of Roldanillo).
The experiment under field conditions
The study was carried out between September 2017 and May 2018 under field conditions, at the farm "La Pola" (04°40' N and 76°10' W, at an altitude of 910 m a.s.l.). Climatic conditions during the releases were as follows: temperature 23.1°C (maximum: 25°C, minimum 20.3°C), relative humidity 81.3% (maximum: 95%, minimum 73%), and a cumulative rainfall of 445.9 mm with a maximum peak of 72.2 mm at 9 weeks after transplanting (WAT).
A randomized complete block design was used, with four treatments and three replicates per treatment. The experimental unit consisted of 16 plants of hybrid papaya Tainung-1, aged between the sixth and 36th week after transplanting, between the phenological stages of vegetative growth and fruit filling. A total of 192 plants were used for the experiment with a distance between plants of 1.5 m and a distance between rows of 3.5 m. Four treatments were established as described in Table 1.
TABLE 1 Description of the treatments for evaluating the control of predators on the populations of Tetranychus urticae in papaya Tainung-1.
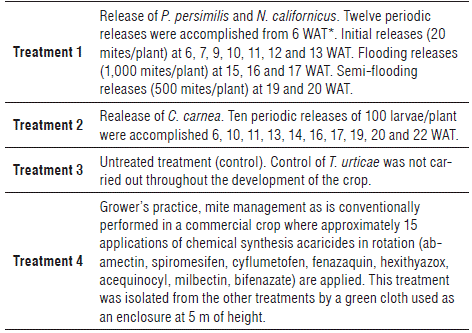
*WAT: Weeks after transplanting.
The predatory mites were released on the leaves of the middle part of the 16 plants in each plot during the morning hours with a mean temperature of 23.1°C and relative humidity of 81.3%. Before opening the bottle, the cap was strongly tapped, since most of the mites were concentrated here, and the bottle was rotated in a horizontal position to allow an adequate mixture of the predatory mites in the vermiculite. The amount and time of release of the Phytoseiidae mites is listed in Table 1, following the recommendations of the producing company and its experience in the release of these Phytoseiidae under controlled conditions. Each bag of C. carnea was hung from each of the 16 plants of the three experimental plots. The release dates of the lacewings are listed in Table 1. The appearance of the first focus of T. urticae on the crop was used as the criterion for the release of predators.
The infestation of T. urticae in the experimental lot was natural for all treatments. However, this was increased early on by the presence of a nearby melon (Cucumis melo L.) crop, which was found infested with the mite, resulting in the experimental lot (mainly block I) being infested. An initial population mean of 7 adults, 39 immatures and 61 eggs per leaf was found for all treatments evaluated by direct counting on papaya leaves. In order to guarantee the highest percentage of hermaphrodite plants in the experimental lot, two plants were planted per site. Once the flowering took place, thinning was performed leaving a single plant, preferably the hermaphrodite plant. The phenological states presented by the culture of papaya are vegetative development (0-11 WAT), beginning of flowering (12 WAT), first fruiting (16 WAT) and reproductive period (more than 30 WAT). Treatments were begun on the sixth week after the transplanting.
Throughout the development of the crop all necessary agronomic work was carried out: irrigation, weed control, disease and insect vectors of diseases, as well as the necessary fertilizations. Plants that had obvious symptoms of virus were removed, but this practice did not affect the experimental design.
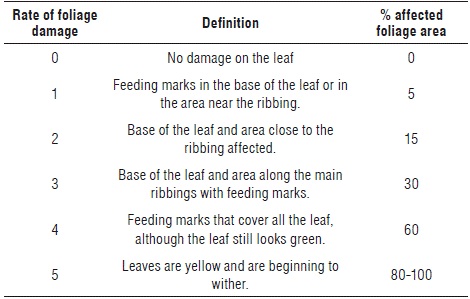
For an evaluation of populations of T. urticae before and after the release of predators as well as the populations of P. persimilis, N. californicus and C. carnea, four plants were randomly tagged in each lot with a weekly monitoring of the incidence of mite damage on the plant (number of leaves with damage/number of total leaves per plant). Leaves considered to be damaged were those that showed obvious chlorosis and food marks. Damage was determined in the inspected leaves using a scale of damage proposed by Tomkiewicz et al. (1993) (Tab. 2), registering the percentage of the foliar area affected.
TABLE 2 Chart of evaluation of Tetranychus urticae's damage (Tomkiewicz et al., 1993). Reproduced with permission of Experimental and Applied AcarologyAcarology.
From each lot, a leaf from the middle part of the plant was collected randomly, and then taken to the Acarology Laboratory of Universidad Nacional de Colombia, Palmira. The populations of each species (eggs, immature and adult stages) were counted with the help of a stereoscope Leica EZ4 8X-35X zoom (Leica Microsystems, Switzerland). Eggs were recognized by their yellowish color and spherical shape, finding them along the leaf veins or above or between the cobwebs. The number of fruits and stem diameters were recorded monthly in order to detect the impact of phytophagous mite populations on crop production. All plants were brought to the first month of harvest and the crop yield was evaluated. Meteorological data were obtained from the RMA ZAR station: Zarzal of Cenicaña.
Data analysis was performed using the SAS 9.3 statistical package (SAS Institute, 2014). Data were subjected to an analysis of variance (ANOVA) and mean separation by least significant difference (LSD) at 5% significance.
Results and discussion
Evaluation of populations of T. urticae and predators
The populations of T. urticae appeared from the fourth week after transplanting (WAT). The presence of a crop of melon (C. melo) at harvest stage located next to the experiment was possibly a focus of the mite pest, increasing the population quickly mainly in block one, for all treatments. The largest populations of T. urticae occurred from the sixth week and increased at the beginning of flowering, reaching the highest peaks between 9 and 20 WAT in all treatments. Populations in T1 and T2 had maximum peaks of 2,699 and 1,862 eggs/leaf respectively. In T3, the populations were very high and reached peaks of 5,482 eggs/leaf. In the T4, the highest peak population was 407 eggs/leaf. When the 19th WAT was reached, the pest mite populations decreased considerably to the point of registering zero mites/leaf in all treatments. The development of T. urticae populations (eggs, immatures, and adults) in each of the treatments is shown in Figure 1.
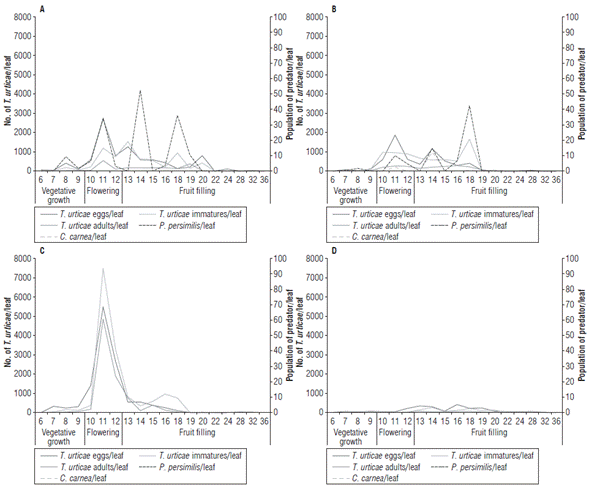
FIGURE 1 Development of the populations of Tetranychus urticae and predators in the different treatments A) T1: Phytoseiulus persimilis and Neoseiulus californicus, B) T2: Chrysoperla carnea, C) T3: Untreated (control) and D) T4: Grower's practice.
In contrast, very few individuals of P. persimilis were found in T1 and T2, with population peaks of 50 and 40 individuals/leaf at 14 and 18 WAT respectively. Once the releases of the Phytoseiidae were finished (19 WAT), the populations practically disappeared. The presence of P.persimilis in T2 can be explained by air currents and the proximity of these treatments in the experiment, especially T1. No individuals of the species N. californicus were recovered in any treatment during all evaluations. Something similar occurred with C. carnea whose populations were very low, only a few individuals were found in T2 (4 eggs/leaf).
Regarding eggs, immatures and adult stages, the untreated T3 showed the largest populations of T. urticae in contrast to the grower's practice T4 that showed the lowest population levels. Eggs of T. urticae per leaf in treatments T1, T2 and T3 showed a significant difference in T4. The immature and adult populations of T. urticae showed no significant difference between T1 and T2 but between T3 and T4 (Fig. 2).
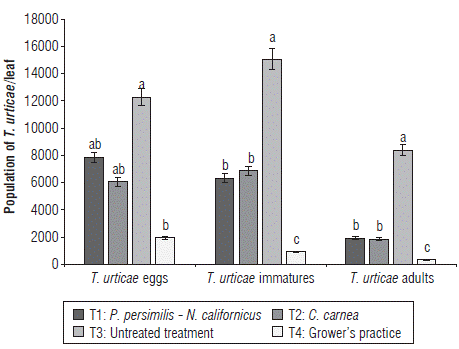
FIGURE 2 Comparison of the population of Tetranychus urticae (eggs, immatures, and adults) in different treatments. Vertical bars indicate the standard error.
For the establishment of Phytoseiidae mites and C. carnea, phytoseiids disperse by walking or flying within a crop (Johnson and Croft, 1976; Hoy et al., 1985; Jung and Croft, 2000). Phytoseiulus persimilis has the longest legs and moves faster than other species, so they move from leaf to leaf, from plant to plant, and can walk a few meters on the ground. These characteristics allow them to ensure a uniform distribution over the plant and adjacent plants, despite the difficulties of flooding releases of phytoseiids on all leaves where the prey is present (Hoy, 2011).
This was not the situation observed in our experiment. In spite of the flooding releases of P. persimilis, N. californicus and C. carnea, no establishment of these natural regulators in C. papaya was accomplished and, therefore, no control effect on T. urticae was observed. Some factors, such as the low initial releases of the predators and the high infestation of T. urticae could have influenced these results. The releases were initiated from plants with an average of nine leaves in which the populations of T. urticae were on average 48 individuals per leaf (between eggs, immatures, and adults). Releases were initiated at a dose of 20 mites/ plant/week approximately at 12 WAT. During this period the maximum number of T. urticae populations was 4,418 individuals per leaf, whereas the maximum number of individuals that P. persimilis showed at that time was 34 individuals between eggs and mobile stages per leaf.
Hoy (2011) indicates that augmented releases of phytoseiids should be performed early when the pest population is low, since these releases usually involve only adult females, which take time to move to adjacent leaves or plants in search of their pray after the releases. Reyes (2012) recommends the use of this predator with flooding releases in papaya for the control of Tetranychus merganser under laboratory conditions, since P. persimilis is not settled when showing a low hatching (5.14 eggs per female) when feeding on this Tetranychidae.
Another aspect that can affect the behavior of Phytoseiidae is the long storage period of predatory mites and C. carnea between the packaging and the releases. The Phytoseiidae were raised in the municipality of Tabio, Cundinamarca and sent by land courier service to the place of the experiment. The storage period was between 48 to 72 h before being released.
When opening the bottle containing the Phytoseiidae, most were located in the lid of the dispenser. This agrees with Daza et al. (2010), who finds that the effectiveness of P. persimilis is diminished by the effect of the trip since it affects the quality and survival of the individuals. Mesa and Duque (1994) indicate the convenience of acclimatizing Phytoseiidae before releases by confining them in cages for a time, as pre-adaptation in the field. In our research, releases were performed directly from the jar with vermiculite to the papaya plants after the long transport.
It is important to consider the architecture of the papaya plant, whose leaves have long petioles, the leaves are distant from each other, and often have drops of latex. It is possible that some of these factors may have disturbed the behavior of the predators. According to Konno et al. (2004), the latex produced by the leaves of C. papaya presents a protease, papain that confers defense against the attack of herbivores, such as polyphagous insects, reducing their rate of growth on that host. Tetranychus urticae, has in its genome some detoxifying genes from bacteria, fungi and plants that it uses to combat the defense mechanisms of the plants it feeds on (Grbic et al., 2011). These could prevent that this papain had a negative effect on this Tetranychidae, but they could have some effect on P. persimilis.
Also, the crop has a leaf blade with a smooth bundle and the underside has protuberant ridges (Arango and Roman 2000), which might prevent the shelter of predators. However, Collier et al. (2007) indicates that T. urticae reared on papaya or green bean is a suitable prey for the development and reproduction of the predatory mite Neoseiulus idaeus under laboratory conditions. This proves that under these conditions the host plant of T. urticae does not affect the predator, guaranteeing the success of N. idaeus as a biological control agent of T. urticae in papaya orchards.
Although there have been very few studies on N. idaeus in papaya crops, it may have the potential to regulate populations of two-spot spider mites in this crop, compared to other Phytoseiidae species. Neoseiulus idaeus is a type II specialized predator that shows substantial adaptations and preference for spider mites. The presence and abundance of N. idaeus throughout the year in commercial orchards with constant acaricide applications indicates that it can develop resistance to pesticides, and this gives more support to its use as a control agent in papaya (Collier et al., 2004).
The period in which the experiment took place coincided with the period of strong winds during the year in the municipality of Roldanillo between October and February with average wind speeds of more than 4.6 km/h (Cedar, 2018). Strong wind could carry predators to other plants different from the ones in which the releases were performed. These winds probably contributed to the rapid dispersion of T. urticae in the experimental plot since this arthropod has aerial-dispersal behaviors that facilitate its escape from the leaf-surface boundary layer and enable it to become airborne. In addition, as wind speed increases, the dispersion response increases (Smitley and Kennedy, 1985).
In relation to the variables stem diameter, number of leaves per plant, percentage of infestation and number, and the weight of fruits per plant, the analysis of variance showed significant differences between treatments (Tab. 3). Mean values were the same for T1, T2 and T3, showing a reduction in the number of leaves/plant and in the number of fruits/ plant and a higher percentage of infestation. In the first month of harvest, the T1 and T2 treatments yielded 58.4% less fruits than the grower's practice, and even though in the T3 a smaller reduction was recorded (50%), the weight of fruits was lower, which is directly related to a lower yield. The grower's practice showed the highest values in the number of leaves per plant and the number and weight per fruit, and the lowest percentage of infestation.
TABLE 3 Variables evaluated to measure the control exerted by predators for the different treatments.
| Treatments | Infestation (%±SEM) | Number of leaves±SEM | Stem diameter (cm±SEM) | Number of fruits*±SEM | Average fruit weight (kg±SEM)* | |
|---|---|---|---|---|---|---|
| T1: | P. persimilis | 78.3±1.3 b1 | 32.9±0.3 ab | 21.2±0.7 b | 2.7±0.8 b | 1.1 ±0.2 b |
| T2: | C. carnea | 80.1 ±1.2 b | 31.7±0.3 b | 21.1 ±0.7 b | 2.6±0.4 b | 1.1±0.1 b |
| T3: | Untreated treatment (control) | 84.3±1.1 a | 30.9±0.3 b | 22.3±0.8 b | 3.2±0.7 b | 0.9±0.2 b |
| T4: | Grower's practice | 61.5±1.1 c | 39.9±0.3 a | 24.5±0.1a | 6.4±0.8 a | 1.5±0.1 a |
1Means followed by equal letters do not differ from each other according to the Tukey's test (P<0.05).
SEM: Standard error of the mean.
*Data collected in the first month of harvest.
The number of leaves is a clear indicator of productivity of papaya, considering that at least one fruit is formed in the bud of each leaf (Storey 1969; Mahouachi et al., 2005). Similarly, the leaves synthesize the carbohydrates that are to be distributed among the different organs of the plant, which allows greater fruit production (Cuéllar and Arrieta, 2010). Thus, a direct relation between the number of leaves and the number of fruits exists, and a decrease in the yield of the crop is observed due to the mites that cause defoliation.
Establishing experiments under real field conditions is important, since the crops grown by the producer entail facing multiple natural factors and problems that cannot be controlled and that do not affect the populations of the pest mite. Therefore, under the conditions of our research the flood releases of predators P.persimilis and N. californicus, and C. carnea were not found to exert control of the T. urticae mite populations. It is possible that some abiotic or biotic factors described above did not allow them to settle down in the papaya crop since the populations of T. urticae in this crop were so high between weeks 6 and 19 after the transplantation that the number of predators released was not enough or these controllers were not released in a timely enough manner. In addition, perhaps some experimental errors could have been made.














