Introduction
The potato (Solanum tuberosum L.) is one of the most important horticultural crops in the world and the fourth most consumed source of carbohydrates for humans after rice, wheat, and corn. Potatoes have high nutritional value and they are consumed in both fresh and processed form (Devaux et al., 2014; Navarre and Pavek, 2015). In Colombia, Diacol Capiro is one of the cultivars with the largest planted areas. It is also the main cultivar (cv.) used for processing due to its high productivity and frying quality (Ñústez, 2011). Dormancy is a factor that can limit seed availability when establishing new crops (Mustefa et al., 2017; Deligios et al., 2020). The most common strategy for breaking dormancy is storage in warehouses (Jansky and Hamernik, 2015). The dormancy period for cv. Diacol Capiro could last up to three months at 15°C and 75% RH (Ñústez, 2011). This affects the costs for seed producers.
The most common potato propagation method is the vegetative one with seed tubers. The quality of seed tubers is linked to different parameters, such as the physiological age of the tuber, its dormancy state and sprouting ability. These are variables that affect the yield potential of the crop (Mikitzel, 1993; Knowles and Knowles, 2006; Struik, 2007; Blauer et al, 2013). Dormancy is the absence of visible sprout growth, even under adequate conditions for it to occur (Suttle, 2007). It is a period that begins simultaneously with the tuber filling at the mother plant and ends with sprouting (Claassens and Vreugdenhil, 2000; Nambara and Marion-Poll, 2005). Dormancy is a plant strategy used to escape from hostile conditions, and this is very important in sessile organisms like plants to ensure their survival (Henis, 1987; Suttle, 2007). In tubers, dormancy is present in the meristems, i.e. buds (Lang et al., 1987; Sonnewald and Sonnewald, 2014), while the rest of the tuber remains metabolically active (Viola et al., 2007; Sergeeva et al., 2012). Viola et al. (2007) establish the presence of at least one sprout of 2 mm length or greater as a criterion to determine the end of the tuber dormancy period. Dormancy breakage begins with the reactivation of the symplastic pathway in the tuber that regulates the distribution of energy reserves towards the meristematic tissues, allowing their activation and sprout development (Viola et al., 2007).
The sprouting process begins with the development of the apical sprout that becomes dominant and inhibits the development of lateral sprouts (Carli et al., 2010). This state is called "apical dominance" or "paradormancy" (Lang et al., 1987; Sonnewald and Sonnewald, 2014). Dominance decreases gradually with the increase of the tuber's physiological age and ends in a state of multiple sprouting that is considered adequate for seed sowing (Krijthe, 1962; Teper-Bamnolker et al., 2012; Blauer et al., 2013; Eshel, 2015). The dormancy period varies according to components, such as genotype, environmental conditions, agronomic management of the crop, storage, and endogenous hormonal dynamics of the tuber (Carli et al., 2010; Sonnewald and Sonnewald, 2014; Eshel, 2015; Christensen et al., 2019; Deligios et al., 2020).
During dormancy the levels of plant growth inhibitors, such as abscisic acid and ethylene, are high. These are the main hormones involved in dormancy initiation and maintenance (Carli et al., 2010; Mani et al., 2014; Sonnewald and Sonnewald, 2014; Deligios et al., 2020). When dormancy is overcome and sprouting begins, the hormonal dynamics change and the concentrations of growth promoters such as cytokinins, gibberellins (Mani et al., 2014) and auxins increase, although the role of auxins is less clear than the other two (Suttle, 2004; Carli et al., 2010).
Different studies have shown that the exogenous contribution of plant growth regulators can increase efficiency of dormancy breakage and potato seed tuber sprouting. The use of exogenous regulators, such as gibberellins (Jansky and Hamernik, 2015; Mustefa et al., 2017; Chindi and Tsegaw, 2019; Christensen et al., 2019; Deligios et al., 2020), cytokinins (Turnbull and Hanke, 1985; Suttle, 2008; Campbell et al., 2014) or their combination (Alexopoulos et al., 2007) have generated positive responses. However, most of the available information is limited to cultivars of the Group Tuberosum, which is not a group of potatoes planted in Colombia (Huaman and Spooner, 2002). The objective of this research was to evaluate the effect of the application of gibberellic acid-3 and 6-benzylaminopurine on tuber dormancy and sprouting in potato cv. Diacol Capiro seed tubers at different immersion times.
Materials and methods
Plant material and experiment establishment
The experiment was carried out at the Universidad Nacional de Colombia Bogota campus, using seed tubers under storage conditions with diffuse light, an average temperature of 16°C and 40-80% relative humidity. Potato cv. Diacol Capiro tubers were used 10 d after harvest. The tubers were washed and classified into three categories (I: 90-110 g, II: 70-89 g, and III: 50-69 g). The tubers that showed morphological changes or some type of damage were discarded.
A completely randomized block design with factorial arrangement (3x3x3) was used. The factors were: gibberellic acid-3 (GA3), 6-benzylaminopurine (6BPA), at concentrations of 0 mg L-1, 25 mg L-1, and 50 mg L-1, and immersion times (iT) of tubers in the solutions at 10, 60, and 120 min. A control without treatment was used for the variable "dormancy period". The experimental unit (EU) included 20 tubers for the variables: dormancy period, weight loss, number of sprouts and secondary sprouting, and five tubers for the variables apical sprout growth, sprout weight and dry matter. Tuber size was used as a blocking factor.
Seed tubers were placed on plastic meshes and submerged in solutions with the different hormone treatments. The tubers were then removed at different immersion times. Subsequently, the tubers were allowed to dry, randomly arranged inside plastic baskets that were stacked in towers per block. The baskets were randomized per height twice a week and stored until the end of the trial, 100 d after applying the treatments (DAT).
Evaluated variables
Dormancy period
The tubers that showed at least one bud with a sprout of 2 mm length or greater were counted and recorded twice a week. With this information, the number of days necessary to obtain 80% of sprouting tubers was determined. This number was considered as the breakage of the dormancy period.
Weight loss and number of sprouts
Tuber weight was determined using a milligram precision balance (XB220A, Precisa, Switzerland) at 8 DAT (initial weight) and 100 DAT (final weight), and the percentage of weight loss was calculated with these data. The number of sprouts was estimated as the total number of sprouts with sufficient vigor to survive in the field at 100 DAT.
Apical sprout growth
Five tubers were randomly selected and the sprouts of the apical zone were individually marked, and their length and diameter measured at 16, 24, 32, 39, 52, 60, 73, and 90 DAT. The tuber growth rate in terms of length and diameter was estimated using the obtained data.
Sprout weight and dry matter percentage
At 100 DAT, all sprouts from five tubers were removed, weighed (fresh weight), and then brought to constant weight in an oven (Thelco Model 16, Precision Scientific Company, Chicago, USA) at 70°C (dry weight). The dry matter percentage was then determined.
Secondary sprouting
The number of tubers that showed secondary sprouting was quantified. The secondary sprouts are characterized by the presence of small and thin sprouts in the primary sprout (Krijthe, 1962).
Statistical analysis
The data were analyzed with the R statistical package (version 3.5.1), using an ANOVA to determine the effect of the factors on parametric variables and the Tukey test for a comparison of means (P≤0.05). For the variable sprout growth (length and width), a generalized linear gamma model with repeated measures was performed based on the methodology of Dobson and Barnett (2008).
Results and discussion
Dormancy period
For this variable, statistically significant differences were observed for GA3, iT and the GA3xiT interaction (Tab. 1).
TABLE 1 Significance levels of ANOVA results of the variables dormancy period (DP), weight loss (WL), number of sprouts (NS), fresh weight of sprouts (FW), dry weight of sprouts (DW), percentage of dry matter of sprouts (% Dm), and secondary sprouting (SS) with the applications of different concentrations of gibberellic acid-3 (Ga3), 6-benzylaminopurine (6BAP), and immersion time (iT) in s. tuberosum cv. Diacol Capiro tubers.
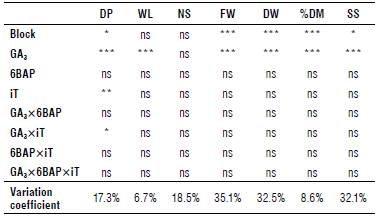
*** P≤0.1%; ** 1%; * 5%; ns: non-significant differences.
The response in the dormancy period was different between GA3 concentrations. Without GA3 application, the dormancy period was 42.2 d on average, whereas with 25 mg L-1 GA3 it was 32.8 d and with 50 mg L-1 GA3 it was 30.1 d. The three levels were statistically different from each other (Fig. 1A). The data showed that GA3 promoted the breakage of tuber dormancy in less time, with the dose of 50 mg L-1 as the most effective.
The dormancy breakage response in the tubers due to the exogenous application of GA3 has been reported in other studies with different cultivars of the Group Tuberosum, such as 'Red Pontiac' (Guzman, 1963), 'Agria' (Salimi et al., 2010; Barani et al., 2013; Mohammadi et al., 2014), 'Marfona' (Salimi et al., 2010; Barani et al., 2013), 'Solara' (Hartmann et al., 2011), 'Draga' (Barani et al., 2013), 'Born' (Mohammadi et al., 2014), 'Bubu', 'Bate' (Mustefa et al., 2017), 'Pasat', 'Dorota' (Wrobel et al., 2017), 'Shepody' (Dean et al., 2018a), 'Spunta', 'Monalisa', 'Europe', 'Arinda' (Deligios et al., 2020) and 'Payette', 'Russet' and 'Alturas' (Dean et al., 2018b). However, the available information in Andigenum cultivars is scarce. Alexopoulos et al. (2007) evaluated treatments with GA3, 6BAP and their combination at different temperatures in the Andigenum cv. Chacasina, applying the solution directly to the tuber-stolon junction. The authors found that GA3 treatments and the combination of GA3 and 6BAP reduced the dormancy period and showed the synergic effect of the two plant growth regulators.
Treatment with iT resulted in statistically significant responses between all the evaluated times as compared to the untreated control (49 d), showing the effect of humidity at the beginning of the sprouting processes (Fig. 1B). The 10 min iT generated a dormancy reduction of 12 d compared to the untreated control. It also differed statistically from 60 and 120 min (14 d), which did not differ from each other (Fig. 1B). These results suggested that, for the cv. Diacol Capiro, 60 min were enough to generate the dormancy breakage response in the evaluated treatments. This result was similar to that reported by Alexopoulos et al. (2008) in the Andigenum cv. Chacasina, in which 120 min was the optimum time of the treatment. It also underlines that the iT has more influence than the plant growth regulator dose, possibly due to the higher amount of solution that enters the tuber. There were interactions between GA3 and iT (Fig. 1C) in which GA3-iT combinations 25-60, 25-120, 50-10, 50-60 and 50-120 reduced the dormancy period by 18 d on average compared to non-treated tubers, and lid compared to tubers immersed in water (Fig. 1C).
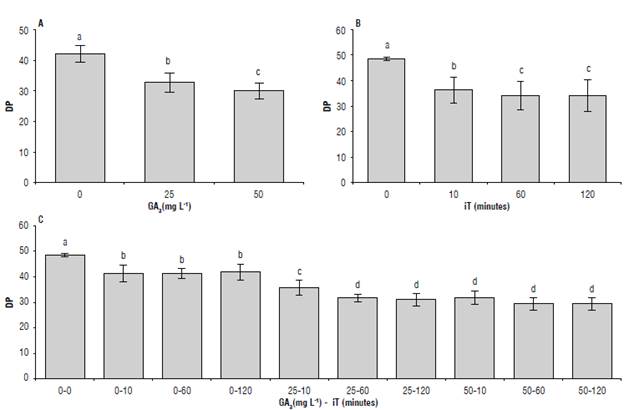
FIGURE 1 Duration of the tuber dormancy period in days (DP) in response to A) gibberellic acid-3 (GA3), B) immersion time (iT), and C) the interaction GA3xiT, in the cv. Diacol Capiro. The data shown is the number of days needed to reach breakage in 80% of the tubers after the application of the treatments. Standard deviations are indicated by vertical bars. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N: A/B = 27; C = 9.

FIGURE 2 Sprout development In A) untreated, B) treated with water for 10 min, C) treated with a 25 mg L1 glbberelllc acld-3 (GA3) solution for 10 min, and D) treated with a 50 mg L1 GA3 solution for 10 min potato (S. tuberosum) cv. Dlacol Capirò tubers 39 days after treatment. Arrows show the buds that have sprouts.
There were no effects of the treatments with 6BAP on the tuber dormancy period. Different studies have shown that the effectiveness of treatments with cytokinins as dormancy disrupters in potato varies with conditions such as tissue sensitivity that is greater in less deep stages of dormancy (Turnbull and Hanke, 1985; Suttle, 2001; Rodriguez and Moreno, 2010; Hartmann et al., 2011), genetic component, and type of cytokinins used (Suttle, 2008).
Weight loss
Only the GA3 treatment showed significant differences in the tuber weight loss (Tab. 1). At 100 d of storage, the treatments with 50 mg L/1 GA3 showed a lower weight loss percentage (4.4%) than the one obtained with 25 and 50 mg L4 (4.9%). The last two had no differences between them (Fig. 3). Weight loss is a response associated with the degree of tuber longevity that increases when sprouting is higher, due to the greater permeability of the cell membranes of the sprouts compared to other tuber tissues (Carli et al., 2010). The response is similar to that reported in cultivars Andigena of the subspecies andigena, such as 'Chacasina' (Alexopoulos et al., 2007; Alexopoulos et al., 2008) and the Group Tuberosum, such as Agria' (Rentzsch et al., 2012), and 'Bubu' and 'Bate' (Mustefa et al., 2017), in which weight loss is related to an increase in the respiratory rate and sprout development in GA-treated tubers.
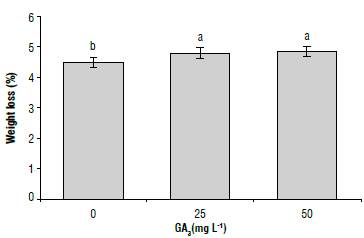
FIGURE 3 Effect of different of glbberellic acld-3 (GA3) concentrations on the weight loss percentage in potato cv. Dlacol Capiro tubers at 100 DAT. Standard deviations are Indicated by the vertical bars. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N = 27.
Number of sprouts
In this study, none of the treatments affected the number of sprouts per tuber (Tab. 1), confirming Alexopoulos et al. (2007). Studies with GA3 applications at low concentrations (20 mg L/1) in cultivars of the subspecies show an increase in the number of vigorous sprouts per tuber (Mustefa et al, 2017). Concentrations between 1 and 2 mg L/1 GA3 in tuber seeds increase the number of stems and the percentage distribution of tubers harvested by size in the crop (Herman et al., 2016; Wrobel et al., 2017; Dean et al, 2018a; Dean et al, 2018b).
Growth in apical sprout length
The applied treatments affected the length of sprouts (Tab. 2). Taking into account the DEVIANCE model used, the differential effect of the DAT (time factor) and their interactions with each of the evaluated factors were identified as follow: GA3xDAT, 6BAPxDAT and TixDAT (Tab. 2). For this reason, the analysis is presented as repeated measures over time.
TABLE 2 Significance levels of DEVIANCE analysis of growth variables in apical sprouts expressed in terms of growth rate in length (L) and diameter (D), treated with different concentrations of gibberellic acid-3 (GA3) and 6-benzylaminopurine (6BAP) and immersion time (iT), in s. tuberosum cv. Diacol Capiro tubers.
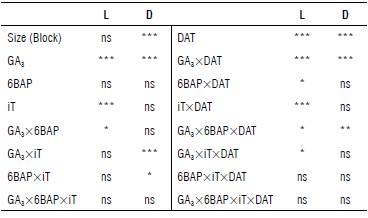
GA3 (Factor): Gibberellic acid-3; 6BAP (Factor): 6-benzylaminopurine; It: Immersion time; DAT (Time factor): Days after treatment; *** P≤0.1%; ** 1%; * 5%; ns: non-significant differences.
The GA3 treatments affected the growth rate of sprouts (Fig. 4A). Treatments with 0 mg L-1 had the lowest growth rate (0.15 mm/d) compared to treatments with 25 mg L-1 (0.26 mm/d) and 50 mg L-1 (0.26 mm/d) without statistically significant differences between the last two. The sprout length at 90 DAT for 50 mg L-1 (2.2 cm) was 83% higher than that in treatments without GA3 (1.2 cm), while in treatments with 25 mg L-1 (2.0 cm) sprout length increased by 66% (Fig. 4B). When observing the effect of 6BAP and iT, GA3 had the greatest effect on the growth of apical sprouts results (Alexopoulos et al., 2008) in the cv. Chacasina.
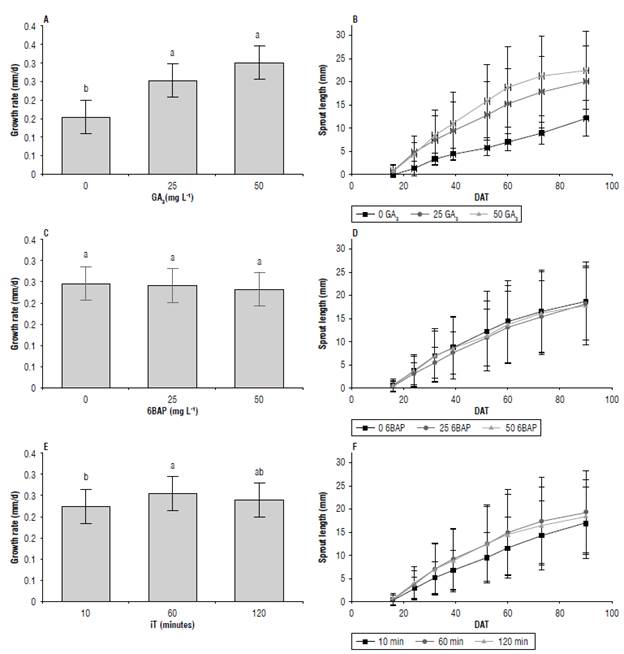
FIGURE 4 Growth rate (A, C, E) and length (B, D, F) of apical sprouts In cv. Dlacol Caplro In response to factors glbberelllc acld-3 (GA3) (A, B), 6-benzylamlnopurlne (6BAP) (C, D) and Immersion time (IT) (E, F) 90 days after treatment (DAT). Data shown In A, C and E correspond to the growth rate during the evaluation period. Vertical bars correspond to the standard deviation. Different letters above the bars indicate a significant difference at 95% confidence, according to the Tukey test. N: A/C/E = 135 per point, B/D/F = 27.
The treatment with 6BPA did not affect the growth rate or length of sprouts during the evaluation period (Figs. 4C and 4D). The factor iT showed differences in sprout length throughout the evaluation time (iTxDAT) (Tab. 2). The growth rate was higher with 60 min (0.25 mm/day) and differed from treatments of 10 min (0.22 mm/d), whereas treatments of 120 min (0.24 mm/d) did not differ from the previous ones (Fig. 4E). However, the length values at 90 DAT were not very different from each other. They showed the lowest value at 10 min (17 mm), followed by 120 min (18 mm) and 60 min (19 mm), with maximum differences of 11% in length (Fig. 4F).
The interaction between GA3xiTxDAT and GA3x6BAPx-DAT affected the length and growth rate of tuber sprouts (Tab. 2). These data suggest that the factor that had the greatest influence was GA3, since treatments with 50 mg L-1 GA3, regardless of their combination with other treatments, generated 3 of the 4 highest sprout lengths (22.6 mm). The highest GA3 concentrations together with the combination 25-60 (GA3-iT) showed no differences in growth rate (Fig. 5A). The other treatments with 25 mg L-1 GA3 (25-120, 2510) resulted in higher sprout length averages (18.5 mm) and higher growth rates than the treatments with 0 mg L-1 GA3 (Fig. 5B). There were no differences in sprout length at 90 DAT or in the growth rate of the treatments with 0 mg L-1 GA3, indicating that the effect of iT requires the presence of GA3 (Fig. 5B).
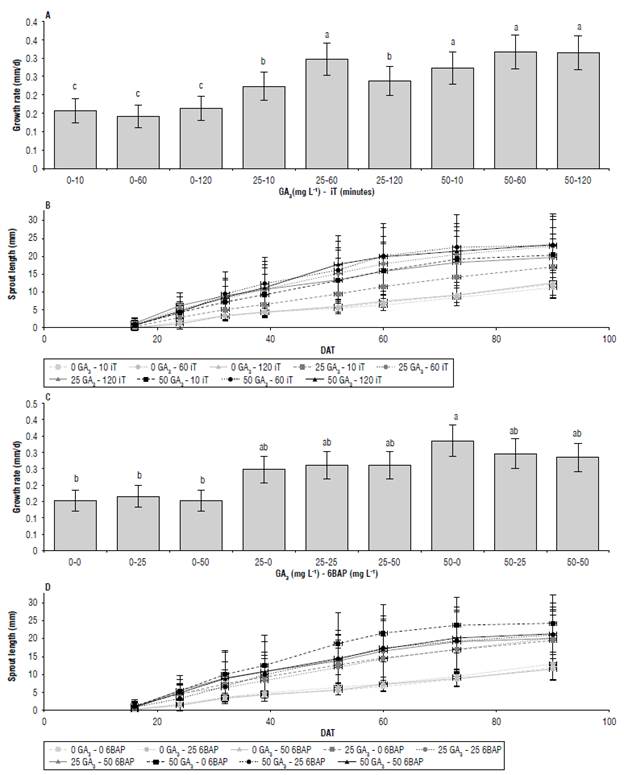
FIGURE 5 Growth rate (A, C) and length (B, D) of apical sprouts In cv. Dlacol Caplro In response to Interactions GA3xlT (A, B) and GA3x6BAP (C, D) 90 days after treatment (DAT). Data shown In A and C correspond to the growth rate during the evaluation period. Vertical bars correspond to the standard deviation. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N: A/C/E = 45 per point; B/D/F = 9.
The GA3x6BAPxDAT interaction showed differences in sprout length at 90 DAT. Only the 50-0 treatment (GA3-6BAP) differed statistically in the growth rate (Fig. 5C) and showed the greatest length (24.5 mm), 49% higher than the average of the treatments with 0 mg L-1 GA3 (12.1 mm) (Fig. 5D).
Growth in diameter of the apical sprout
With the statistical model used, the effects of DAT and its interaction with GA3 and the interaction GA3x6BPA (Tab. 2) were identified. The GA3 affected the diameter growth rate of the sprouts. The treatments with 0 mg L-1 had the highest growth rate (0.073 mm/d), significantly higher compared to 50 mg L-1 (0.045 mm/d) (Fig. 6A). At the end of the evaluation (90 d), the application of 50 mg L-1 GA3 generated smaller sprout diameter at levels 25 and 0 mg L-1, respectively (Fig. 6B). This coincides with Alexopoulos et al. (2008).
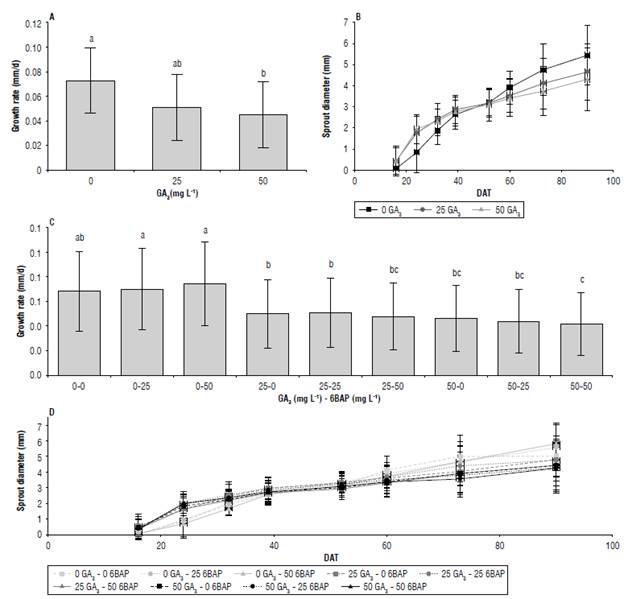
FIGURE 6 Growth rate (A, C) and diameter (B, D) of apical sprouts in the cv. Dlacol Caplro in response to glbberellic acld-3 (GA3) (A, B) and the GA3x6BAP Interaction (C, D) 90 days after treatment (DAT). Data shown In A and C correspond to the growth rate during the evaluation period. Vertical bars correspond to the standard deviation. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N: A = 135 per point, B = 27; C = 45 per point; D = 9.
We saw no interaction between GA3 and 6BAP in the sprout diameter, but we observed the growth rate as a product of the interaction of the factors GA3x6BAPxDAT (Tab. 2, Fig. 6C). The treatment with 0 mg L-1 GA3 and with 6BAP did not differ from the treatment with 0 mg L-1 GA3 and without 6BAP in the diameter growth rate (Fig. 6C), but the diameter in length was superior to the other treatments with different GA3 and 6BAP combinations. This result suggests a negative effect of GA3 on sprout diameter (Fig. 6D), but a positive effect on length.
Jansky and Hamernik (2015) observed similar results in the growth of sprouts in tubers treated with GA3. They found increases in sprout elongation with doses of up to 1000 mg L-1 GA3 and attributed this effect to the role this plant growth regulator plays in cellular elongation processes. The development of long and/or thin sprouts is a characteristic that can affect the quality of the tuber as seed by making sprouts more susceptible to damage by handling during transport and planting (Virtanen et al., 2013).
Sprout weight and dry matter
Significant differences were only found for the growth of sprouts (Tab. 1, Fig. 7) represented by fresh weight (FW), dry weight (DW) and dry matter percentage (DM) in response to GA3 application. The FW and DW of the sprouts were significantly lower for treatments with 0 mg L4 GA3 (3.02 g, 0.68 g) compared to 25 mg L4 (4.06 g, 0.87 g) and 50 mg L/1 (4.54 g, 0.95 g), but there were no significant differences between these last two (Fig. 8A, Fig. 8B). These results are similar to those observed by other authors in different cultivars (Alexopoulos et ah, 2007; Salimi et al, 2010; MustefaefaL, 2017).

FIGURE 7 Apical sprouts of potato (S. tuberosum) tubers cv. Dlacol Capirò A) immersed In pure water for 10 min, B) treated with 25 mg L1 glbbe-rellic acid-3 (GA3) for 60 min, and C) treated with 50 mg L1 GA3for 120 min, 90 d after treatment.
The dry matter percentage in the sprouts showed significant differences for the treatments with GA3 (Fig. 8C). Treatments with 0 mg L/1 GA3 obtained greater dry matter percentages (23%) than the treatments with 25 mg L/1 and 50 mg L4 (21%) with no differences between the last two. These results suggest that sprout growth promoted by GA3 was caused by cellular elongation rather than by division. Salimi et al. (2010) find similar results in response to GA3 treatments. However, partially opposite results are reported by Alexopoulos et al. (2007), who find that tuber sprouts treated with GA3 and 6BAP are larger, with greater dry mass percentages than in untreated tubers.
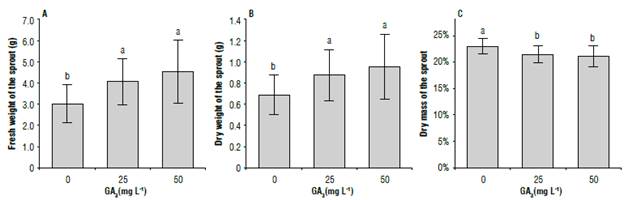
FIGURE 8 A) Fresh weight, B) dry weight, and C) percentage of dry matter of tuber sprouts of S. tuberosum L. cv. Dlacol Caplro in response to different doses of glbberellic acld-3 (GA3). Vertical bars correspond to the standard deviation. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N: A/B/C = 27.
Secondary sprouting
Secondary sprouting corresponds to the formation of thin sprouts that grow on the main sprout (Krijthe, 1962). A significant effect of GA3 was observed in our research (Tab. 1), increasing the percentage of occurrence when the dose was higher, with results of 51% for treatments with 0 mg L/1, 80% for 25 mg L4 and 88% for 50 mg L/1 (Fig. 9). Secondary sprouting takes place in tubers with very long periods of storage time and this is associated with tuber senescence (Struik, 2007; Carli et al., 2010) or high storage humidity.
Blocking in the experiment was performed by tuber size and this influenced most of the variables, because size is associated with the physiological age of the tubers (Struik, 2007; Salimi et al., 2010; Mohammadi et al., 2014; Christensen et al, 2019). The dormancy period decreases when the tuber is larger (Knowles and Knowles, 2006). In our research, the largest tubers developed larger sprouts with a higher dry matter content and increased secondary sprouting. These data show that there is a differential response to treatments with growth regulators due to the size of the tubers, which highlights the importance of taking this factor into account to apply GA3 as a seed treatment.
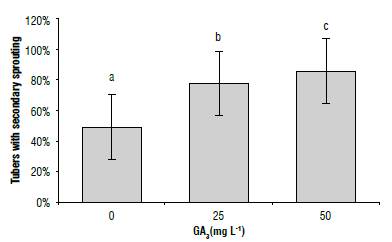
FIGURE 9 Percentage of tubers with secondary sprouting In S. tuberosum L. cv. Dlacol Caplro treated with different concentrations of glbberellic acld-3 (GA3). Standard deviations are Indicated by vertical bars. Different letters above the bars indicate significant difference at 95% confidence, according to the Tukey test. N = 27.
Conclusions
The results showed that exogenous GA3 applications decrease the dormancy period of cv. Diacol Capiro tubers and their effect increases as an effect of prolonged immersion times (60 and 120 min). The treatments with 25 or 50 mg L-1 GA3 and iT 60 min reduced the dormancy period by 35% (18 d). However, 50 mg L-1 GA3 generated long sprouts with smaller diameter and lower DM content. This makes them more susceptible to mechanical damage and increases the occurrence of characteristics associated with advanced physiological age and affects the quality of the tuber. In this study, 6BAP showed no clear effect on dormancy breakage.














