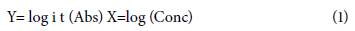Introduction
The study of mycotoxins has been an object of interest for the world in the last twenty years, not only because of the large losses in crop and animal productivity but also because of their deleterious effects. Mycotoxins are considered the secondary metabolic product of a large number of fungi that in small amounts are capable of triggering alterations and pathological conditions in both humans and animals. The intake of food contaminated with mycotoxins may cause acute or chronic mycotoxicosis. Depending on the degree of toxicity, the central nervous, digestive, cardiovascular, and pulmonary systems can be affected.
Mycotoxins can also trigger cancerous diseases, produce mutagenic and teratogenic damages and, in some cases, can behave as immunosuppressants (Bennett and Klich, 2003; Bertero et at., 2018).
Mycotoxins are produced by fungi capable of growing on a wide variety of substrates, so they often contaminate food. The biosynthesis is performed by a few precursors derived from intermediates of its primary metabolism. Production occurs during the stationary phase of growth with little or no production of these mycotoxins during the active growth phase (Berthiller et at., 2013; Pitt and Miller, 2017).
Since mycotoxins were recognized as a public health problem, quantification by analytical techniques has continuously improved. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are the most used analytical techniques today. These techniques determine several trichothecenes simultaneously with low concentration levels (ng g-1) of evaluated samples, requiring high stringency, especially in the cleaning processes. The ELISA technique allows a large number of samples to be measured routinely with high precision compared to CG and HPLC methods, which are expensive and take longer to get results (Escobar and Fragas, 2004; Pleadin et al., 2012).
One of the first reports on food contamination by Fusarium mycotoxins in human and animal health mentions the T-2 toxin produced by alimentary toxic aleukia (ATA), which was reported in Russia in the 1940s and fumonisins that cause equine leukoencephalomalacia, reported in several countries in the 1980s (Pitt and Miller, 2017).
Mycotoxin production is stimulated by oxidative and nutritional stress, environmental factors such as pH, temperature, water activity, fungicides and secondary metabolites produced in these environments (Martínez et al., 2010). Evaluations in some regions of Europe showed that 80-100% of grains were contaminated with trichothecenes (Reverberi et al., 2010). These high incidences occurred in regions where crops were affected by drought, infested by insects, or inadequately maintained using inappropriate harvesting equipment or storage facilities (Pohland, 1993).
In the case of Fusarium, which is essentially regarded as a field pathogen, mycotoxins are synthesized mainly during plant infection; in the case of cereals they can accumulate in the grain during the production, harvesting and storage stages (Desjardins and Hohn, 1997). Species of this genus, which are contaminants of these plants, can produce a large variety of mycotoxins. The mycotoxigenic profile may vary at inter- and intra-specific levels and often includes a wide range of mycotoxins. Fumonisins and trichothecenes are the most frequent and toxic my-cotoxins produced by Fusarium, although this genus can produce other important mycotoxins such as zearalenone, enanthines, and moniliformina (Chakrabarti, 2013; Zhang et at., 2013).
Environmental conditions, susceptible hosts, and virulence of the pathogen are the factors that promote fungus growth and, consequently, the production of toxins. Therefore, agricultural products affected by these microorganisms may be susceptible to contamination in the production, harvesting, transport, and storage processes (Nelson et at., 1993; Desjardins and Hohn, 1997; Desjardins and Proctor, 2007; Zhang et at., 2013).
In Colombia, resolution 4506 of October 30 establishes the maximum levels of food contaminants intended for human consumption (Ministry of Health and Social Protection, 2013). The maximum permissible limit for deoxynivalenol (DON) is 100-1750 ng kg-1, for fumonisins (FUM) it is 200-4000 ng kg-1 and for zearalenone (ZEA) it is 20-350 ng kg-1. These ranges depend on the food type, the size of particles generated in the industrial process, and the age of the consumers. These values are remarkably similar to those managed by the European Union (EU) and Mercosur (WHO/FAO, 2003).
The overall objective of this study was to quantify the mycotoxins present in Fusarium isolates from a collection of samples belonging to the Universidad Nacional de Colombia located in Palmira (Valle).
Materials and methods
This study was conducted in the laboratory of Plant diagnostics of the Universidad Nacional de Colombia located in Palmira.
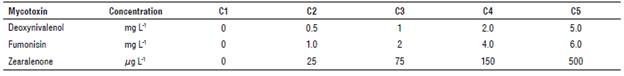
Fourteen isolates previously evaluated by PCR (Tab. 1) with the trichothecene (TRI), fumonisin (FUM) and zearalenone (PKS) genes were selected and amplified for trichothecene (DON), fumonisin (FUM) and zearalenone (ZEA) detection (Tab. 2).
TABLE 1 Primers used to detect mycotoxins in Fusarium isolates from the collection of the Universidad Nacional de Colombia - Palmira campus.
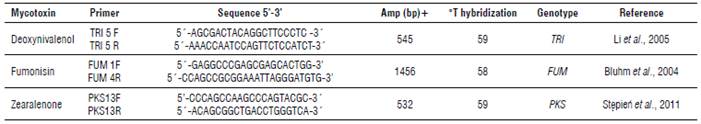
Amp (bp)+ = expected amplicon size in base pairs.
TABLE 2 Mycotoxin detection present in isolates obtained by phylogenetic analysis from the collection of the Universidad Nacional de Colombia ■ Palmira campus.
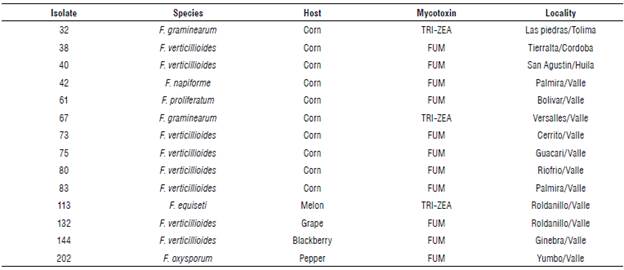
*TRI: Trichothecene; FUM: Fumonisin; ZEA: Zearalenone.
Mycotoxin quantification methods
The isolates were cultured in Petri dishes with potato dextrose agar (PDA) medium. Then, to determine the amount of mycotoxin, the isolates were introduced to corn kernel medium with a concentration of1 x 108 ml-1 conidia. These isolates were preserved and incubated with a photoperiod of 12 h of light and 12 h of darkness. Once the mycelium reached growth for 15 d, the conidia count was carried out in a Neubauer chamber at a concentration of 1 x 106 ml-1 conidia; then, it was separated with a stirring rod adding 10 ml of 70% methanol. The mix was then filtered with Watman No. 1 paper and 100 nl of the solution was added with a micropipette to the controls and samples following the protocol of the NEOGEN® Veratox commercial kit according to the established concentration for each myco-toxin. Finally, the reading was performed in a Neogen 4700 microwell reader 9303 (NEOGEN© Corporation, Lansing, MI, USA) with a length of 650 nm wave. Calibration curves for the quantification of deoxynivalenol, zearalenone and fumonisins were performed with the controls established for each kit (Tab. 3).
Statistical analysis
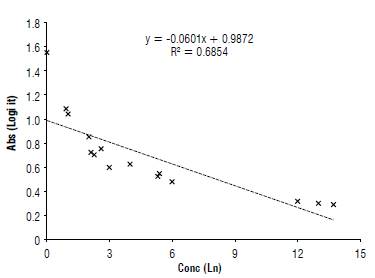
The absorbance values and the concentration of the samples were performed using the regression formula established by the Elisa reader program (Eq. 1) (NEOGEN© Corporation, Acumedia, Lansing, MI, USA) through the optical densities of both the controls and the samples to be evaluated.
where X and Y are the axes.
Results and discussion
Deoxynivalenol
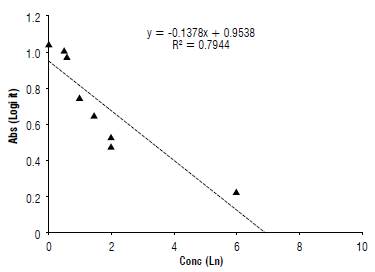
The results show that the evaluated isolates (32, 67, and 113) have concentrations ranging between 0.6 and 2 mg L-1, corresponding to F. graminearum and F. equisetti (Fig.1). This method demonstrates there is a high sensitivity for the test that is the most used for trichothecene detection in different foods for both human and animal consumption (Meneely et al., 2011).
Studies of trichothecene quantification are performed using methods universally accepted by the International Official Association of Analytical Chemists (AOAC International), recognized by the Food and Drug Administration (FDA) for the determination of mycotoxins (Yoshizawa et al, 2004).
The results obtained in this evaluation indicate that the values for the presence of trichothecenes in food for human consumption are above the levels allowed in Colombia and the EU (Rojas and Wilches, 2011). Values greater than 750 [ig kg4 (0.75 mg kg4) found in F. graminearum isolates in corn indicate a high mycotoxin concentration in the evaluated samples. In Argentina corn samples with a high incidence of DON contamination of 0.93 mg kg4 have been found (Pacin et ah, 1997). In Brazil, the values of this toxin did not exceed 0.6 mg kg4 (Furlong et ah, 1995). There are no universal values for measuring the damage caused by DON in foodstuffs for human consumption. However, each country or group of countries, such as the EU and Mercosur, manage ranges between 300-2000 μg kg4 with the lowest values being those of the EU (Pleadin et ah, 2012).
In Colombia, studies on the detection and quantification of DON have been recorded since 1995 (Diaz and Cespedes, 1997; Duarte and Villamil, 2006; Rojas and Wilches, 2011; Rojas et ah, 2015). These studies show a high concentration of DON in foodstuffs for human consumption, especially processed products from corn and wheat. This fact is worrying since to date controls and application of normativity are not carried out in the Colombia despite the resolution of the Ministry of Health and Social Protection issued in 2014 (Ministry of Health and Social Protection, 2013).
Risks caused by DON are initiated when it enters the food chain, and it is transmitted to humans directly through consumption of cereals and cereal products. Foods such as corn and wheat are the most susceptible to be contaminated with DON. The toxin can remain in processed foods made from contaminated cereal, such as bread, pasta, cookies, etc. Toxicity may occur by contact (causing skin, eye and throat irritation) or by direct ingestion. Depending on the amount ingested, it may cause vomiting, diarrhea, tachycardia, leucopenia (immunosuppressive effect due to leukocyte reduction), or teratogenic effects (associated with congenital diseases) (Sudakin, 2003; Pietsch et ah, 2014; Yang et ah, 2014).
On the other hand, it is important to highlight that trichothecenes such as DON are translocated in the plant tissues before any symptoms of the disease and signs of the fungus occur. Metabolites act by decreasing the plant protein synthesis and are able to suppress or delay the defense response. It has been found that isolates that do not produce trichothecenes are less pathogenic and affect the productive development of the plant to a lesser extent (Desjardins and Hohn, 1997; Desjardins, 2006).
Zearalenone
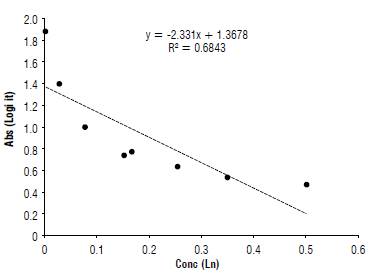
In isolates 32 and 67 (F. graminearum) and 113 (F. equiseti) amplified with the PKS gene by PCR, there was also the presence of mycotoxin zearalenone through the serological ELISA test (Fig. 3). F. graminearum predominates in environments with temperatures of 25°C and relative humidity greater than 88%. In contrast, F. equiseti develops in tropical and subtropical areas affecting several crops, appearing mainly in harvest and post-harvest time. Several studies show that zearalenone production in stored food increases with water activity, i.e., the amount of free water that is available for microorganisms' growth. The optimum amount is 0.98 mg kg4 which facilitates toxin biosynthesis (Lacey and Magan, 1991, Velluti et ah, 2000).
The maximum permissible values of this mycotoxin in human food are 0.1 mgkg4 (Ministry of Health and Social Protection, 2013). However, the values found in the evaluated isolates are above the established ranges for Colombia (Fig. 2).
It is observed that the DON and ZEA mycotoxins appear simultaneously in the species F. graminearum and F. equiseti, and this condition is further aggravated due to the possible synergistic effects associated with ingestion of food contaminated with various mycotoxins. In Kenya, the simultaneous ocurrence of DON, ZEA, OTA (ochratoxin) and AFs (aflatoxins) obtained from wheat crops has been reported (Muthomi et al, 2008; Li et al, 2014).
In studies conducted by Rojas et al. (2015) on mycotoxin co-occurrence in food for infants, it was found that ZEA shows high concentrations between 421.34 and 1518.22 μg kg1 (0.421-1.5 mg kg4), which exceed the legal values established for Colombia and the EU (20 μg kg4). In a study carried out in 1999 (Diaz, 2005), values ranging from 35 to 134 μg kg4 (0.035-0.134 mg kg4) were found in corn, which are above the current ranges, as well as those found in this study.
The zearalenone toxin is thermostable which allows it to survive under adverse conditions. Once it enters the body it is rapidly metabolized, producing estrogenic substances such as β-zearalenol and (3-zearalenol. These substances have in their structure a lactone that initiates estrogenic activity capable of competing and interacting with estrogen receptors which activate and deactivate metabolic pathways (Li etal, 2014).
Information about damages caused by ZEA in humans is poor. However, studies concerning high ZEA concentrations in foods and the emergence of diseases associated with estrogen such as precocious puberty and breast cancer have led to the belief that this toxin triggers a series of events that cause the formation of cancer cells and also affects the immune system (EFSA, 2011).
The World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) categorize ZEA and DON produced by F. graminearum into group 3, i.e. they are not classified as carcinogenic to humans (IARC, 2016). However, damage caused by these toxins to humans is reflected in estrogenic damage (Rojas and Wilches, 2011).
Fumonisin
High concentrations of FUM were found in F. verticillioides isolates 38,40, 73 and 75 from corn exceeding the control values of 6 mg kg4 (Fig. 3). In Colombia, the permitted limits for this toxin are 3 mg kg4; however, for the EU the values range from 0.2 to 4 mg kg4. For the species F. proliferation values also exceed the permitted ranges. In the species F. napiforme, F. oxysporum and F. verticillioides (isolates 80, 83 and 144), the concentration of FUM does not exceed those ranges.
Since corn is a daily food in Colombia, concentrations found in culture medium are high compared to the estimated ranges for direct and processed food. Studies carried out in Mexico, where FUM concentration in corn both under field and controlled conditions were evaluated, a high amount of fumonisins (5 mg kg4) is found above the established standards, becoming a potential risk for human and animal health (Gallardo-Reyes et al., 2006). In a study conducted in Brazil by Ono et al. (1999) high relative humidity and temperature are key to fungus growth and subsequent fumonisin contamination in the field. The period close to cob maturity or grain filling generates the highest FUM levels (Martinez et al., 2010). Dry periods before and during the grain filling also promote the disease severity and increase the fumonisins accumulation (Munkvold, 2003).
Fumonisins are also produced by some species of the F. fujikuroi species complex (FFCS) such as F. proliferatum and F. napiforme and the F. oxysporum species complex, with the toxin amount playing an important role in plant pathogenicity (Winter et al., 1996; Braun and Wink, 2018). FUM formation is caused by the expression of the FUM gene (Waalwijkef al, 2004; Stępień et al, 2011). The FUM gene cluster is highly correlated between F. oxysporum, F. verticillioides and F. proliferatum given by number of genes, orientation and order (Proctor et al., 2008). FUM FBI amount depends on the substrate, the genotype and the environmental conditions; these conditions play an important role in the transcription factors of the FUM gene (Jurado et al, 2008).
The species F. verticillioides is well known worldwide as the largest FUM producer. In addition, toxicity in corn has been documented for more than 100 years (Desjardins and Hohn, 1997). Studies about FUM toxicity were initiated in 1988 when a devastating disease occurred affecting farm animals especially horses, donkeys, mules and rabbits. Research in South Africa determined that F. verticillioides was the causal agent of leukoencephalomacia. This research led to conduct relevant studies with this mycotoxin, demonstrating that oral and intravenous doses lead to the disease development in horses and liver cancer in rats (Marassas, 1988).
The World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) categorize this mycotoxin into group 2B, i.e., possibly carcinogenic. However, it is believed that there is a relationship between the occurrence of F. verticillioides and esophageal cancer (IARC, 2016; WHO/FAO, 2019). Epidemiological studies conducted in Italy, Iran, Zimbawue, China, the United States and Brazil relate this type of cancer to high concentrations of FUM B1, B2 produced by F. verticillioides. Additionally, the high intake of corn and wheat in diets with low content of minerals such as manganese, molybdenum, selenium, folate and vitamins A, C, E and B12 may also promote disease development (Cao et al., 2013). In regions such as China, South Africa, and the Texas-Mexico border, neural tube defects are associated with high corn intake (Mutchinick et al., 1999; Gueant-Rodriguez et al., 2006).
For Colombia, mycotoxin detection studies are required in food for direct consumption, raw materials and processed products to generate the necessary information to determine the safety of the food consumed.
The ELISA technique used in this study allowed the quantification of mycotoxins DON, ZEA and FUM in the isolates previously detected by PCR.
The high levels of mycotoxins detected and quantified with the extraction method used and the immunoabsorption test allowed the obtention of permissibility levels above those established for Colombia.