Introduction
The potato (Solanum tuberosum L.) is considered the fourth most important crop in terms of planted area and the third most consumed food by humans. This tuber plays a key role in food security and constitutes a high portion of the human diet in developing countries (Ezekiel et al., 2013; Devaux et al., 2014; FAO, 2018). Potato tubers are consumed worldwide and are cooked in different ways. However, eating unpeeled boiled potatoes is predominant in many regions (Camire et al., 2009).
Potatoes contain a wide range of phytochemicals such as phenolic acids, anthocyanins and carotenoids, and diploid potatoes known as the Phureja Group are considered an important source of these phytochemicals (Pillai et al., 2013; Narvaez-Cuenca et al., 2018). Hydroxycinnamic acids are the predominant acids in potatoes, and within these, chlorogenic acid is the most abundant (Mattila and Hellstrom, 2007; Piñeros et al., 2017). Anthocyanins are water-soluble phenolic compounds that belong to the flavonoid group and are responsible for the red, blue and purple color in fruits and vegetables (Fang, 2015; Tierno et al., 2015). They are also present in the skin and flesh of potato tubers (Brown, 2008). Carotenoids are lipophilic pigments responsible for the yellow, orange, and red colors in vegetables (Rodriguez-Amaya, 2018). These phytochemical compounds are metabolites with antioxidant activity that protects cells from oxidative stress damage, play an important role in the tuber organoleptic properties and provide multiple benefits for the human body (Craft et al., 2012; Ezekiel et al., 2013; Bellumori et al., 2017).
The concentration of these compounds with antioxidant activity in potato tubers is affected by factors such as the genotype, environment, agronomic crop management, and postharvest processes (André et al., 2009; Ezekiel et al., 2013; Lachman et al., 2016). Among the agronomic factors, fertilization is important because a balanced management of nutrients ensures that plants can reach their genetic potential reflected in the quality and yield parameters (Gómez, 2005).
There is scarce information about the effect of plant nutrition on the content of phenolics and carotenoids or their antioxidant activity in potato tubers. In Colombia, research on macro and micronutrients has been conducted solely to analyze their effect on yield and quality of commercial potato cultivars (Gómez et al., 2006; Palacios et al., 2008).
Magnesium (Mg) and manganese (Mn) are two of the essential plant nutrients involved in multiple biological functions (Farzadfar et al., 2017). Although these elements have been studied in different topics such as tuber yield and quality (Pérez et al., 2008; Villa et al., 2011), there are no reports in connection with their antioxidant activity.
The purpose of this research was to evaluate the effect of foliar applications of magnesium and manganese on the antioxidant activity, total phenol, total anthocyanins, phenolic acids, and carotenoid contents of four diploid potato cultivars, Criolla Colombia, Paola, Milagros and Violeta.
Materials and methods
Plant material
Tubers of diploid potato cultivars with different colors of skin and flesh were used for this study. The cultivars Criolla Colombia and Paola had yellow skin and flesh; the cultivar Milagros had red skin and yellow flesh, and the cultivar Violeta had purple skin and purple and white flesh (Fig. 1).
Chemicals
Ethanol, methanol, Folin Ciocalteu reagent, 2,2'-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), acetate trihydrate, iron(III) chloride hexahydrate, glacial acetic acid, chlorhidric acid, sodium carbonate and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) were purchased from Panreac. Gallic acid was obtained from Alfa. The 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and standards of zeaxanthin, antheraxanthin and lutein were obtained from Sigma Aldrich. Acetonitrile, butyl hydroxytoluene (BHT), hexane, and triethylamine were obtained from J.T. Baker. Standards of chlorogenic acid and caffeic acid were obtained from Chromadex. Standards of neochlorogenic acid and cryptochlorogenic acid were obtained from PhytoLab. Water was obtained by a Millipore system (EMD Millipore Corp, Billerica, MA, USA).
Foliar fertilization with magnesium and manganese
The field experiment was conducted at the experimental farm Centro Agropecuario Marengo of the Universidad Nacional de Colombia, located in Mosquera, Cundinamarca, Colombia (4°41'04.44" N, 74°12'57.46" W, 2543 m a.s.l.) with an annual average precipitation of 750 mm and annual average temperature of 14°C, from October 2016 to January 2017. The characteristics of the soil used in the experiment are shown in Table 1. The experimental design consisted of a randomized block with three replicates. Four treatments were evaluated: 1) control (without Mg or Mn applications), 2) 450 g ha-1 of Mg, 3) 300 g ha-1 of Mn, and 4) combination of elements, 450 g ha-1 of Mg and 300 g ha-1 of Mn. The experimental unit was 66 plants per cultivar. Applications were split into three foliar sprays, at 60, 75 and 90 d after planting. The source of Mg and Mn used were chelates (EDTA). Basal fertilizer [10-20-20 (N-P2O5-K2O)] was applied at a rate of 25 g per seed tuber at sowing. Potato tubers were harvested at 120 d after planting and a sample of each replicate was collected for cooking.
Cooking process
For each treatment, four tubers of approximately similar size (40-50 g) were selected. Whole and unpeeled tubers were washed with tap water and placed in a stainless-steel pot containing boiling water. Cooking time tests were performed on each cultivar by piercing the tubers with a knife. The following cooking times were used: 20 min for cultivars Criolla Colombia and Paola and 19 min for cultivars Milagros and Violeta. After cooking, the samples were cooled at room temperature, placed in sealed plastic bags and frozen at -80°C. The samples were then freeze-dried and pulverized with a blender. Each sample was stored in an amber glass flask at room temperature until use.
Extraction of phenolic compounds
Phenolic compounds were extracted using the methods reported by Piñeros et al. (2017) and Burgos et al. (2013). Fifty mg of lyophilized sample was extracted with 1 ml of methanol/water/acetic (50:50:0.1, v/v/v) using sonication at 4°C for 10 min. The mixture was centrifuged at 5000 rpm at 4°C for 15 min and the supernatant was collected. Extraction was performed five times. The five supernatants were mixed, flushed with nitrogen gas, and stored at -20°C until further analyses.
Determination of total phenols
Total phenol content was determined using the Folin Ciocalteu method (Waterhouse, 2002). An aliquot 20 of the extract solution was taken into a cuvette, and then 1.58 ml of deionized water was added. After 8 min at room temperature, 300 of a sodium carbonate solution (20%, w/v) was added, and the mixture was left to stand for 90 min in the dark at room temperature. Absorbance was measured at 765 nm with a spectrophotometer (SmartSpec Plus, BIORAD, Richmond, CA, USA). The total phenol concentration was calculated using a gallic acid calibration curve (r2 = 0.9984) ranging from 10 to 100 ml-1. Data were expressed as mg of gallic acid equivalents per 100 g of potato dry weight (DW).
Determination of antioxidant activity
Antioxidant activity was determined by ABTS and Ferric ion Reducing Antioxidant Power (FRAP) assays. For ABTS assays, the procedure was carried out according to the method of Re et al. (1999) with some modifications. The radical ABTS was activated by mixing 2.5 mM of a potassium persulfate solution and 7 mM of an ABTS solution in equal quantities and allowing them to react for 16 h in the dark. The solution was then diluted with 99% ethanol to obtain an absorbance of 0.70 at 734 nm using a spectrophotometer.
Briefly, 10 of each phenolic extract sample was added to 1 ml of ABTS solution into a cuvette. Absorbance was measured at 734 nm. The antioxidant activity was calculated using a trolox acid calibration curve (r2 = 0.9975) ranging from 50 to 800 μM. Data were expressed as of trolox equivalents per 100 g of potato DW.
The FRAP assay was carried out according to Benzie and Strain (1996) with some modifications. The FRAP solution was obtained by mixing 50 ml of 300 mM acetate buffer, pH 3.6; 5 ml of10 mM TPTZ solution, 5 ml of FeCl3-6H2O and 2.4 ml of deionized water. Briefly, 30 of each phenolic extract sample were added to 90 of deionized water and allowed to react with 900 of FRAP solution for 5 min in a cuvette. Absorbance was measured at 593 nm with a spectrophotometer. The antioxidant activity was calculated using a trolox acid calibration curve (r2 = 0.9975) ranging from 50 to 800 μM. Data were expressed as of trolox equivalents per 100 g of potato DW.
Determination of total anthocyanins
Total anthocyanin content was determined according to Burgos et al. (2014). For anthocyanin extraction of cultivars Criolla Colombia, Paola and Milagros 40 mg of lyophilized sample and 20 mg for cultivar Violeta were used. Lyophili-zed samples were extracted with 1 ml of a methanol/1.5 M chloridric acid (80:20, v/v) solution using sonication at 4°C for 5 min and placed in a water bath at 80°C for 5 min. The mixture was centrifuged at 5000 rpm at 4°C for 5 min and the supernatant was collected. Extraction was performed four times for cultivars Criolla Colombia, Paola and Milagros, and six times for cultivar Violeta. The supernatants were mixed and stored at -20°C until analysis.
Then, 1.5 ml of extract was taken into a cuvette. Absorbance was measured at 700, 545 and 515 nm with a spectrophotometer. Total anthocyanin concentration was calculated using as reference the extinction coefficient (3.02 x 104) and the molecular weight (718.5 g L-1) of malvidin-3-p-coumarylglycoside for the sample of cultivar Violeta and the extinction coefficient (2.73 x 104) and the molecular weight (486.5 g L-1) of pelargonidin-3-glucoside for samples of cultivars Criolla Colombia, Paola and Milagros. Data were expressed as mg of anthocyanin per 100 g of potato DW.
Determination of phenolic acids
The phenolic extracts were filtered through disposable nylon syringe filter units (0.2 μm, Thermo Scientific, Waltham, MA, USA).
The phenolic extract was analyzed according to Narvaez et al. (2013). Separation was performed using a Hypersil gold RP-C18 column (150 mm x 2.1 mm, 1.9 μm, Thermo Scientific). The mobile phase was composed of water/ acetonitrile/acetic acid (99:1:0.1, v/v/v) (solvent A) and acetonitrile/acetic acid (100:0.1, v/v) (solvent B). The elution program consisted of isocratic conditions at 0% B for 5 min; linear gradient from 0 to 60% B for 18 min, and from 60 to 100% B for 1 min; isocratic conditions at 100% B for 3 min; linear gradient from 100 to 0% B for 1 min, and conditioning in isocratic starting conditions at 0% B for 7 min. The volume of injection was 5 μl. The flow rate was 400 μl/min. Detection was performed at 325 nm.
Identification was based on the comparison of retention time and UV spectra of standards. Quantification of chlorogenic acid, cryptochlorogenic acid, and neochlorogenic acid were based on the calibration curve with chlorogenic acid ranging from 0.1 to 10 mg L-1 (r2 = 0.9956). Quantification of caffeic acid was performed by using a calibration curve with caffeic acid ranging from 0.05 to 10 mg L-1 (r2 = 0.9994). The results were expressed as mg 100 g-1 of potato DW.
Determination of carotenoids
Carotenoids were identified and quantified by reversed phase (RP) - ultrahigh performance liquid chromatography (UHPLC) with diode array detection (DAD) (Dionex Ultimate 3000, Thermo Scientific, Waltham, MA, USA).
Extraction was carried out according to Maurer et al. (2014). Under dim light conditions, 250 mg of lyophilized sample were weighed into an amber plastic centrifuge tube and 2 ml of acetone/methanol (2:1, v/v), containing 0.5% (w/v) butylhydroxytoluene (BHT), were added, followed by 1 ml of hexane. The mixture was sonicated at 4°C for 20 min and 1.5 ml of cold 1 mol L-1 aqueous sodium chloride was added to the extract. The mixture was centrifuged at 5000 rpm at 4°C for 10 min. The supernatant was collected and stored at 4°C. Extraction was performed three times. The three supernatants were mixed and concentrated to 1 ml at 20°C under a nitrogen gas atmosphere. The concentrated extract was filtered through a disposable nylon syringe filter unit (0.2 μm, Thermo Scientific, Waltham, MA, USA) and analyzed by RP-UHPLC-DAD. Separation was performed using an AQUITY UPLC RP18 column (150 mm x 2.1 mm, 1.7 μm, Waters, Milford, MA, USA) with a temperature of 20°C. The mobile phase was composed of ethyl acetate/triethylamine (100:0.25, v/v) (solvent A), acetonitrile/triethylamine (100:0.25, v/v) (solvent B), and acetonitrile/water/triethylamine (50:50:0.25, v/v/v) (solvent C). The elution program consisted of isocratic conditions at 95% B for 1 min; linear gradient from 95 to 65% B for 12 min; isocratic conditions at 65% B for 2.5 min; linear gradient from 65 to 95% B for 2 min; and conditioning in isocratic starting conditions at 95% B for 9.5 min. The volume of injection was 5 μ1. The flow rate was 250 μl/min. Detection was performed at 450 nm.
Zeaxanthin, antheraxanthin, and lutein were identified in the extracts by comparing their retention times and UV-Vis spectrum against external standards. Quantification of each carotenoid was based on calibration curves of standards ranging from 1 to 100 mg L/1 (r2≥0.9993). Results were expressed as mg 100 g4 of potato DW.
Results
Total phenolic content
The foliar fertilization with Mg (450 g ha-1) had a significant effect on the cultivar Criolla Colombia, contrasting with cultivars Milagros and Violeta that showed a reduction when compared to the control. There was no effect on the cultivar Paola. The foliar fertilization with Mn (300 g ha-1) had a significant effect only on the cultivar Milagros reducing the total phenolic content when compared to the control. The two elements in combination did not show a significant effect on any of the cultivars. The cultivar Violeta showed the highest mean value for the total phenolic content and it was significantly higher than the cultivars Criolla Colombia and Paola (Tab. 2).
TABLE 2 Antioxidant activity, phenolic compounds, and carotenoids of cooked potato tubers of four diploid cultivars with Mg and Mn foliar fertilization.
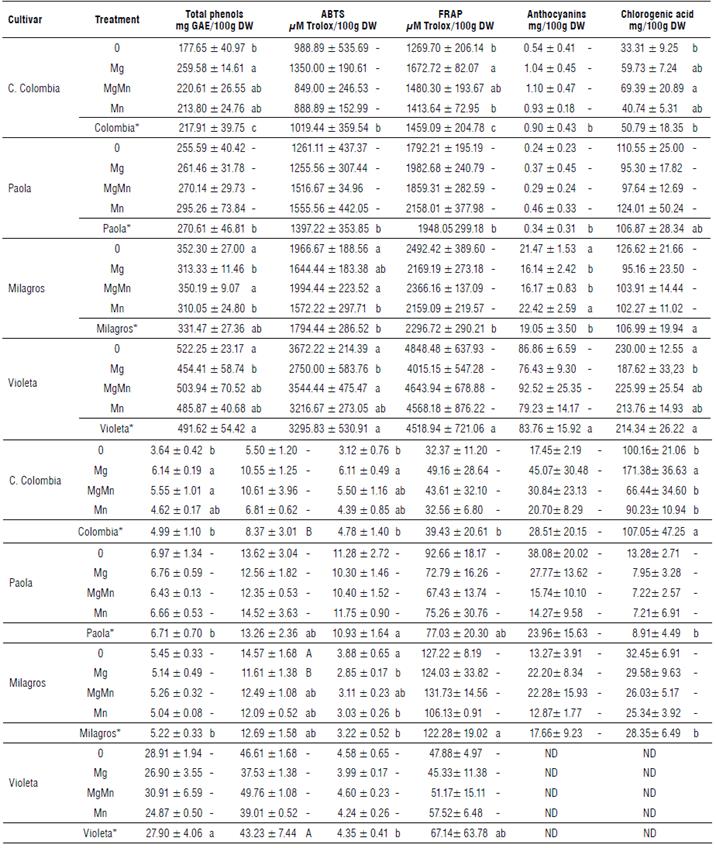
Treatments: 0 (withoutfertilization), Mg (450 g ha-1), MgMn (450 and 300 g ha-1), and Mn (300 g ha-1). ABTS (2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid), FRAP (Ferric ion Reducing Antioxidant Power). Different letters after the mean values indicate significant differences by the Tukeytest (P< 0.05) ± Sd. "Cultivar mean. ND: Not detected.
Antioxidant activity by the ABTS method
The foliar fertilization had a significant effect on the cultivars Milagros and Violeta while the other cultivars did not show a response. These significant effects were observed with Mn (300 g ha-1) and Mg (450 g ha-1), respectively, that led to a reduction in the antioxidant activity when compared to the control treatment. The cultivar Violeta had the highest average value and was significantly superior to the other cultivars (Tab. 2).
Antioxidant activity by the FRAP method
The foliar fertilization had a significant effect on the cultivar Criolla Colombia. The fertilization with Mg (450 g ha-1) increased the antioxidant activity compared to the control. The cultivar Violeta showed the highest mean value compared to the cultivars Criolla Colombia, Paola and Milagros (Tab. 2).
Total anthocyanin content
The foliar fertilization had a significant effect on the cultivar Milagros with Mg (450 g ha-1) and Mg and Mn (450 and 300 g ha-1) compared to the control, showing a reduction in the anthocyanin content. There were no significant effects of the foliar fertilizer application on the rest of cultivars. The cultivar Violeta showed the highest mean value for the total anthocyanin content among all cultivars (Tab. 2).
Phenolic acid content
Figure 2 shows a chromatogram with the results for the quantification of phenolic acids with the UHPLC. There was a retention time of 9.64 min for the neochlorogenic acid, 11.31 min for the chlorogenic acid, 11.56 min for the cryptochlorogenic acid, and 11.87 min for the caffeic acid. To generate the chromatogram, data were collected from samples of the cultivar Violeta as a control (no treatment with Mg and Mn). A range from 9 to 13 minutes was considered as this range provides the highest peaks for the acids under study.
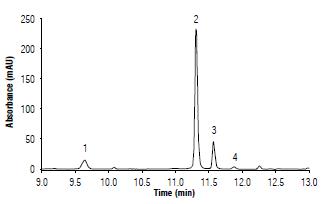
FIGURE 2 Chromatogram of phenolic acids quantified in boiled potato tubers from cultivar Violeta recorded at 325 nm. 1: Neochlorogenic acid; 2: Chlorogenic acid; 3: Cryptochlorogenic acid, and 4: Caffeic acid.
The foliar fertilization had a significant effect on the cultivar Criolla Colombia with the treatment Mg and Mn (450 and 300 g ha-1) showing an increase in the content of chlorogenic acid and neochlorogenic acid compared to the control. Similarly, the treatment Mg (450 g ha-1) significantly increased the neochlorogenic and caffeic acid contents compared to the control. In cultivar Milagros, the Mg (450 g ha-1) significantly decreased the cryptochlorogenic acid and caffeic acid content compared to the control. The same effect was observed with the Mn (300 g ha-1) on the caffeic acid content. No significant effect from the fertilization treatment was observed on the cultivar Paola (Tab. 2), and only a significant reduction in the chlorogenic acid was observed in the cultivar Violeta when it was treated with 450 g ha-1 of Mg.
From all quantified acids, chlorogenic acid was predominant. The cultivar Violeta showed the highest mean values for chlorogenic, neochlorogenic and cryptochlorogenic acids. These values were significantly higher when compared to the other cultivars. In the case of the caffeic acid, the cultivar Paola showed the highest mean value, statistically higher among all cultivars (Tab. 2).
Carotenoid content
Figure 3 shows a chromatogram with the results for the quantification of carotenoids with the UHPLC. There was a retention time of 3.67 min for antheraxanthin, 4.52 min for lutein, and 4.81 min for zeaxanthin. To generate the chromatogram, data were collected from the samples of the cultivar Criolla Colombia as a control (no treatment with Mg and Mn). A range from 3 to 5 min was considered as this range provided the highest peaks for the carotenoids under study.
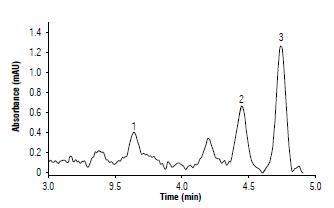
FIGURE 3 Chromatogram of the carotenoids quantified in boiled potato tubers from cultivar Criolla Colombia recorded at 425 nm. 1: antheraxanthin, 2: lutein and 3: zeaxanthin.
Lutein content did not show a significant effect from the fertilization treatments compared to the control in the four cultivars evaluated. Cultivar Milagros showed the highest mean value for lutein, but it was not significantly different from the cultivar Criolla Colombia, which showed the lowest mean value (Tab. 2).
There was no significant effect of the treatments on the content of antheraxanthin in the cultivars Criolla Colombia, Milagros and Paola. These cultivars showed similar mean values without significant differences. This carotenoid was not detected in the cultivar Violeta (Tab. 2).
There was a significant effect of fertilization with Mg (450 g ha-1) on the content of zeaxanthin in the cultivar Criolla Colombia compared to the control, showing an increase in the carotenoid. In cultivars Paola and Milagros, the treatments did not have a significant effect, while in cultivar Violeta it was not detected (Tab. 2).
Discussion
There is very little information about the effect of foliar fertilization with magnesium and manganese in potato. However, documented research findings on fertilization with magnesium in potato plants are in line with the responses observed in this study for the cultivars Milagros and Violeta. Klein et al. (1982) found that the fertilization of potato crops with this element significantly reduced the content of phenols and suggests a possible effect of the phenolase enzymes. Despite not finding significant differences, Hamouz et al. (2006) observe that the content of phenolic compounds decreased with magnesium fertilization. Phenolase enzymes such as polyphenol oxidase are associated with the degradation of anthocyanins and oxidation of phenolic compounds in plants (Ruenroengklin et al., 2009). In the potato, these enzymes are present in the tubers (Tian et al., 2016).
The effect of manganese has been reported in other species with a possible action in the activity of phenolase enzymes. Farzadfar et al. (2017) evaluated the activity of the enzyme polyphenol oxidase in Tanacetum parthenium plants fertilized with this element. The results showed a reduction in the activity of the enzyme related to a greater content of phenolic compounds; however, in this study, Mn did not show an effect on these compounds in the four cultivars evaluated.
From the treatment responses observed, this research emphasizes the important influence of the genotype, as reported by many authors who attributed a wide variation in genotypic differences of antioxidant activity, phenolic compounds, and carotenoids in potato (Andre et al., 2007; Reddivari et al., 2007; Lachman et al., 2012; Tierno et al., 2016; Oertel et al., 2017; Cuéllar-Cepeda et al., 2019).
The responses observed in this study showed a greater influence of the genotype on the treatments applied, as has been reported by numerous authors who attribute the differences in antioxidant activity and the content of phenolic and carotenoid compounds in potatoes to a wide genotypic variation (Andre et al., 2007; Reddivari et al., 2007; Lachman et al., 2012; Tierno et al., 2016; Oertel et al., 2017; Cuéllar-Cepeda et al., 2019).
The observed higher content of total phenols and antioxi-dant activity in the cultivars Milagros and Violeta compared to cultivars Criolla Colombia and Paola, correspond with the studies of Lachman et al. (2012), Navarre et al. (2011) and Rytel et al. (2014). These studies record values between two and three times higher in red and purple potato tubers compared to yellow tubers. The high antioxidant activity observed in the cultivar Violet coincides with the reports of Reyes et al. (2005); Lachman et al. (2008); Valiñas et al. (2017) and Cerón-Lasso et al. (2018), who show that varieties of potato with purple flesh contain higher amounts of antioxidant compounds, where anthocyanins are the most representative.
There was a high variation in the concentration of phenolic acids among the cultivars evaluated. However, chlorogenic acid exhibited a greater content in all cultivars. These results coincide with the studies by Narváez-Cuenca et al. (2013) in whole potato tubers of commercial cultivars of Colombia, Piñeros et al. (2017) in cooked potato tubers of different varieties and cultivars of Phureja Group of Colombia, Rytel et al. (2014) in processed yellow and colored potato tubers of Czech Republic, and Gutiérrez-Quequezana et al. (2020) in whole purple potato tubers of Switzerland. The total contents of chlorogenic, cryptochlorogenic, and neochlorogenic acids were lower in the cultivars Criolla Colombia, Paola and Milagros than in the cultivar Violeta that attained values between two and five times higher. These results are consistent with those reported by Ezekiel et al. (2013) and Bellumori et al. (2017), who found a much higher concentration of phenolic acids in purple potato tubers.
The carotenoid content showed variation among cultivars. In the cultivars of yellow flesh ('Criolla Colombia', 'Paola' and 'Milagros') lutein, antheraxanthin, and zeaxanthin were detected and quantified. In the cultivar Violeta, only lutein was detected. These responses are coincident with Tatarowska et al. (2019) and Kotíková et al. (2016), where the content and type of carotenoids detected were different between cultivars. The higher contents of carotenoids were associated with yellow cultivars compared with the purple ones, and the presence of only lutein in a cultivar similar to Violet (skin and flesh of purple color) was reported.
Conclusions
The responses of the evaluated variables to the treatments applied were different for each cultivar, showing an important effect of the genotype.
Foliar fertilization with Mg had an effect on total phenol content and antioxidant activity. These values increased in cultivar Criolla Colombia whereas they decreased in cultivar Violeta.
Foliar fertilization with Mn reduced the antioxidant activity by the ABTS method and the content of total phenols and caffeic acid in the cultivar Milagros. No significant effects were observed in the other cultivars.
The content of carotenoids showed high variability among the cultivars. In the cultivar Violeta (purple skin and flesh), contents of antheraxanthin and zeaxanthin were not detected, while lutein was detected in all cultivars with similar contents.
Foliar application of Mg and Mn cannot be recommended as a general agronomic practice if the purpose is to increase the content of phenolic compounds, carotenoids and antioxidant activity in cultivars of the Phureja Group, as there are genotype-specific responses.
This study allows a suggestion for the use of the cultivar Violeta in further studies to determine in vivo antioxidant activity. This is a reference cultivar for diploid potato breeding regarding characteristics associated with antioxidant activity.
















