Introduction
The sugarcane aphid Melanaphis sacchari/sorghi (Zehntner, 1897) (Hemiptera: Aphidididae) is native to the African continent and has become an invasive pest attacking sorghum cultivation in the Americas (Zapata et al., 2016). In 2013, it arrived in Mexico causing losses between 30 and 100% (Rodríguez-del-Bosque & Terán, 2015; Knutson et al., 2016). It is considered a "super-clone" due to its low genetic diversity, but it has great potential for adaptation to various geographical and climatic regions (Nibouche et al., 2018; Perales-Rosas et al., 2019). This aphid feeds on phloem causing direct damage to the plant, and like most aphids it excretes honeydew. Honeydew is the result of excess sugar in the aphid's diet and is used as a food source by many other organisms. This results in the formation of black sooty mold that can reduce the photosynthetic capacity of the sorghum plant (Peña-Martínez et al., 2018).
Different strategies have been implemented for the management of this pest, such as the delimitation of sowing dates, conservation of natural enemies, use of biological control, elimination of alternate hosts, etc. (Knutson et al., 2016; De Souza & Davis, 2019). However, chemical control has become the primary control measure (Bowling et al., 2016; Knutson et al., 2016). Crop nutrition as a management tactic for M. sacchari/sorghi has not had much relevance, due to the scarce information on the subject. It is well-known that the availability of numerous nutrients affects the development and survival of herbivorous insects, since their biomass generally contains much higher concentrations of elements compared to plants (Boswell et al., 2008). Nitrogen (N) fertilization strongly influences aphid population growth parameters; increased nitrogen is associated with higher nymph growth rates and, thus, increases the fecundity of many aphid species (Hosseini et al., 2010; Chow et al., 2011; Gash, 2012). Increased nitrogen fertilization can improve the nutritional quality and plant attractiveness for aphids by improving the dietary parameters of the phloem with an increase in the level of amino acids and nitrates in the host plants (Douglas, 2006; Fallahpour et al., 2015). Recent studies have shown a marked increase in the population parameters of M. sacchari/sorghi with increased nitrogen fertilization (Lama et al., 2019; Wilson et al, 2020).
To date, no work has been reported on the relationship between potassium and phosphate fertilization with the population dynamics of M. sacchari/sorghi. However, it is known that potassium (K)-induced changes in plant metabolite concentrations are multiple and include potassium dependence on enzyme metabolism (including those associated with jasmonic acid and salicylic acid), photosynthesis, and long-range transport (Amtmann et al., 2008). According to Venter et al. (2014), the application of potassium phosphate to wheat crops induces a certain degree of tolerance to the attack of the Russian wheat aphid (Diuraphis noxia). Phosphorus (P) can act as a host plant susceptibility modifier by changing secondary metabolites such as phenols and terpenes. When this occurs, the accumulation of phenolic compounds (tannins, lignin) acts as a barrier that has deterrent or directly toxic effects on herbivorous insects (Facknath & Lalljee, 2005). In some cases, such as those reported by Sinha et al. (2018), P produced a repressive effect on the insect population, where the mustard aphid (Lipaphis erysimi) considerably decreased its population by applying high doses of this element.
The present study was carried out under the hypothesis that the nutritional imbalance in the sorghum crop influences the population density of M. sacchari/sorghi. Based on this hypothesis, the objective of this research was to determine the relationship between N, P and K and the populations of M. sacchari/sorghi (Hemiptera: Aphididae).
Materials and methods
Location and preparation of host plants
The experiment was conducted in a greenhouse of the Department of Agricultural Parasitology at the Universidad Autonoma Chapingo. In the establishment of the crop, seeds of hybrid sorghum UPM-219 were used, which has a high susceptibility to M. sacchari/sorghi attack. So, it was possible to observe the relationship between fertilization and the aphid population without the interference of other factors such as weather, natural enemies, etc.
Sorghum seeds were sown in polyethylene bags with a capacity of 6 L, which were filled with soil from the experimental field. This soil has a clay loam structure, with a content of 3.4% organic matter, 6.99 mg kg-1 of phosphorus Bray, 363 mg kg-1 of potassium and 5.33 mg kg-1 of nitrogen as nitrate. Although N and P levels are considered low to medium for this type of soil, the K levels are considered medium to high. Five sorghum seeds were placed in each bag; 5 d after emergence the plants were thinned out, keeping the two most vigorous and largest plants. The irrigation kept the soil at levels close to field capacity, to maintain the optimal development of the crop.
Evaluated treatments
Five applications of fertilizer were carried out, the first at the time of sowing and the remaining every 7 d. This was done with the intention of minimizing the toxic effects of fertilizers on plants. The fertilizers were weighed with an Ohaus Adventurer® precision analytical balance and mixed in individual plastic bags and were applied manually in diluted water (solution). The fertilizers used were urea (46% N) as a source of N, Ca3(PO4)2 (calcium phosphate) as a source of P, and KNO3 (potassium nitrate) as a source of K, applied in three levels, based on previous analysis:
Low level: half of the required dose.
Medium level: optimal dose.
High level: double the required dose.
The treatments consisted of combinations of the three nutrients mentioned above. For N and P three levels of fertilization were used (high, medium and low), whereas for K only two levels were used (high and medium), this because of the concentrations of the nutrient found in the soil. As an experimental unit, a 6 L plastic bag with two sorghum plants was used. For the calculation of the nutrient dose, the nutritional needs for a production of 8000 kg ha-1 and a density of 180,000 plants ha-1 were taken into account as proposed by Fontanetto et al. (2009). Regarding the final dose of fertilizers, the pre-existing concentrations of nutrients in the soil used for the study were considered, adding the necessary amounts of fertilizer needed to reach the stable dose. The experimental design used was a randomized whole block design with 12 treatments and six replicates. The treatments are described in Table 1.
Plant infestation
The plants were infested with 15 nymphs of M. sacchari/ sorghi in the third instar (the number of nymphs was determined based on preliminary trials, where 15 nymphs were an optimal number for the development of the population) within 60 d of emergence of the crop. The nymphs used for the infestation were obtained from a purification brood; five adult females (obtained from an infested sorghum crop) were placed on 5 cm diameter circles of sorghum leaves, which were previously placed in Petri dishes with agar to maintain the turgidity of the leaves. These boxes were taken to a bioclimatic breeding chamber for 3 d (25 ± 1°C, R.H. 60 ± 5% and a 12:12 photoperiod). Twenty-four hours after the beginning of breeding, the adult females were removed and when the infestation of the plants took place, the breeding was homogenized leaving 15 third-instar nymphs in each circle.
Estimation of the density of M. sacchari/sorghi in the different treatments
The rapid monitoring scale proposed by Bowling et al. (2015) was used to estimate the aphid population. This consists of a diagrammatic scale that allows us to determine approximate numbers of aphids per leaf through images and averages. One lower and one upper leaf of each plant per pot were evaluated, performing a total of six samples at seven-day intervals, starting 7 d after infestation.
Biochemical components of sorghum
Two samples of leaf tissues were collected, one at the vegetative stage and the other at full bloom. Total soluble sugars were measured by the anthrone method proposed by Witham et al. (1971). Absorbance was measured at 600 nm with a Spectrophotometer (Bausch & Lomb, Rochester, NY, USA), and reducing sugars were measured by Somogyi's method (Somogyi,1952), measuring absorbance at 540 nm in the spectrophotometer. Total soluble proteins were quantified using Bradford's method (Bradford, 1976) measuring absorbance at 595 nm in the spectrophotometer. Nitrogen, phosphorus and potassium were also analyzed. For N, the microKjeldahl method (Bremner, 1965) was used. For P, the molybdenum blue method (Murphy & Riley, 1962) for color development was used and absorbance was measured at 420 nm in a Spectrum-20 Spectrophotometer (Bausch & Lomb, Rochester, NY, USA). Potassium was determined by a flammometry Photometer Corning 400 (Corning Inc., NY, USA), and Mg content was measured by atomic absorption in Phyllis Pye SP9 Spectrophotometer (Pye Unicam Ltd. Cambridge, England).
Data analysis
The population data of M. sacchari/sorghi were subjected to an analysis of variance and means comparison test with the Tukey's method (α = 0.05). The statistical analyses were performed using a generalized linear model using the GLIMMIX procedure. Since aphids have a contagion distribution, a Poisson distribution was considered as a link function to the natural logarithm. For the parameters of N, P, K, Mg, total soluble proteins, total soluble sugars and reducing sugars, the general model was used with the General Linear Model procedure. These analyses were performed with the SAS® software (Statistical Analysis System) version 9.4.
Results
Relationship between N, P and K fertilization with the M. sacchari/sorghi population
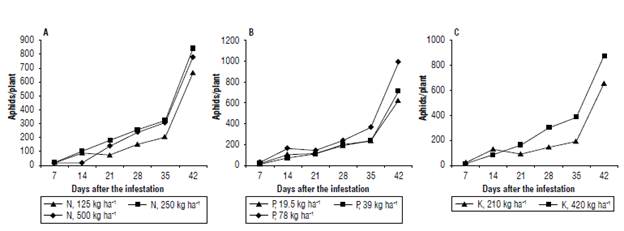
Figure 1 shows that the sugarcane aphid population is a function of the different levels of applied macroelements N, P and K. Nitrogen was the element that had a significant effect (P<0.0001) on the increase of the sugarcane aphid population, showing a positive correlation; in other words, the more nitrogen applied, the greater the density of aphids. This behavior has an arithmetical trend, given that when the N goes from 125 to 259 kg ha-1, the aphid density almost doubles. However, after 259 kg ha-1 the density of the sugarcane aphid showed a slight reduction that maybe due to intraspecific competition (Applebaum & Heifetz, 1999). According to Honek (1993), as the insect population increases, nutritional resources decrease, which results in a drop in fertility due to the smaller size of the females.
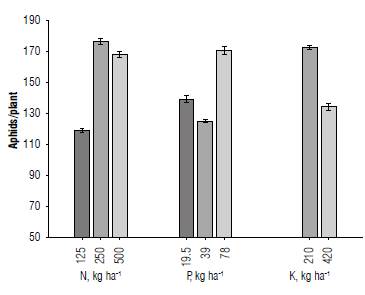
FIGURE 1 Relationship between N, P and K fertilization and M. sacchari/ sorghi population. Bars indicate the standard error (SE).
Contrary to the two previous elements, when the dose of potassium is doubled, a considerable reduction is observed in the number of aphids estimated per plant. Figure 2 shows the evolution of the sugarcane aphid population in response to different levels of fertilization, where a gradual increase in the upper levels of N and P fertilization is evident (Fig. 2 A-B), while the opposite was observed in the case of K (Fig. 2 C).
Effect of N, P and K fertilization on some biochemical components of sorghum leaves
In Tables 2 and 3, the results of the foliar analyses showed that N doses had a positive correlation with leaf N concentration. Similar results were obtained with P concentrations in response to P doses and K concentrations in response to K doses on both assessment dates. K, Mg, total soluble proteins, and total soluble sugar concentrations showed significant differences in the different doses of nitrogen applied, with a positive correlation with magnesium and total soluble proteins, and vice versa with K and total soluble sugars. However, for P and reducing sugar concentrations, nitrogen applications were not significant.
TABLE 2 Effect of N, P and K on biochemical components at the vegetative stage of sorghum.
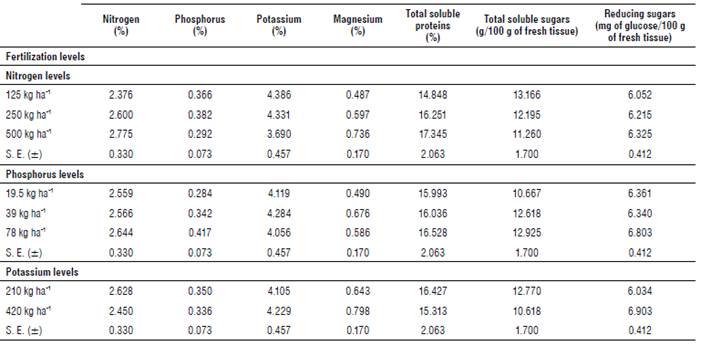
S. E. = Standard Error of the mean.
TABLE 3 Effect of N, P and K on biochemical components at the reproductive stage of sorghum.
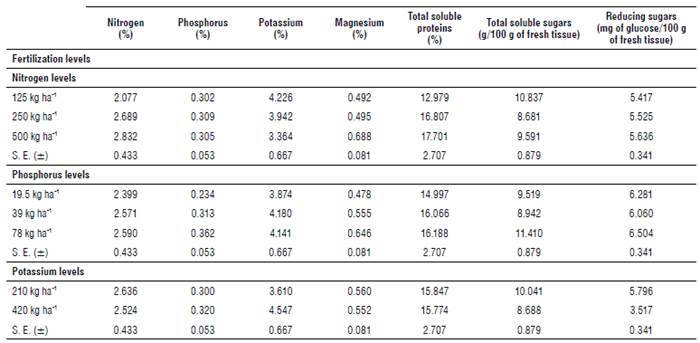
S. E. = Standard Error of the mean.
In both evaluations, phosphorus applications were not significant for N, K, total soluble protein and reducing sugar concentrations; however, the addition of phosphorus to the crop generated a significant effect on Mg and total soluble sugar concentrations, with a positive correlation in both cases.
In the case of phosphorus, a positive relationship with the sugarcane aphid density is observed, given that when the dose of P is increased, the density of the pest increases (P<0.0001), that is, when the population increases by 36% from 39 kg ha-1 to 78 kg ha-1.
Potassium applications to the crop showed a significant effect on the concentrations of reducing sugars and total soluble sugars, where a marked decrease in these can be observed with the increase of the dose of potassium.
As for total soluble protein concentrations, Mg, N and P concentrations, potassium application was not significant (Tabs. 2 and 3). It can also be seen that in the second evaluation, all the biochemical components considered showed a decrease in their concentrations.
Relationships between the M. sacchari/sorghi population and biochemical components in sorghum leaves
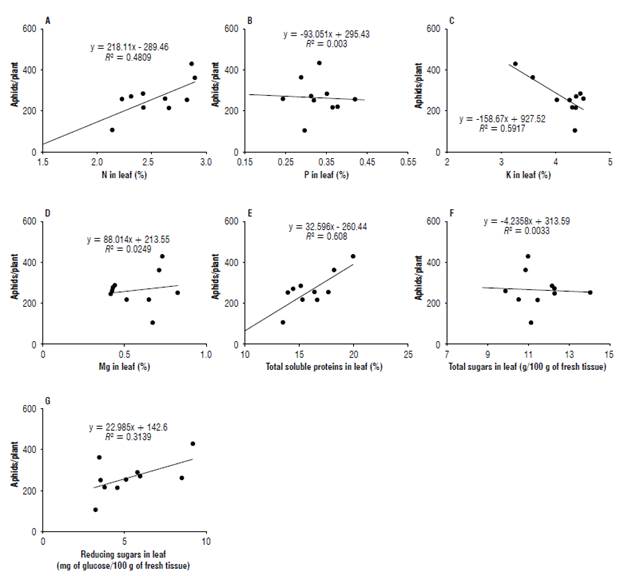
Figure 3 summarizes all the relationships between the sugarcane aphid populations and the biochemical components of sorghum (N, P, K, Mg, total soluble proteins, total soluble sugars and reducing sugars). The sugarcane aphid population was positively correlated with N (r = 0.6935), total soluble protein (r = 0.7797) and reducing sugar (r = 0.5602) concentrations, but it was negatively correlated with K (r = -0.7692) content in sorghum leaves.
Discussion
Fertilization is one of the most important pillars in agricultural production, and sorghum is no exception. The essential nutrients N, P and K that are applied to sorghum in different fertilizer forms maintain soil fertility and prevent deficiencies of these nutrients from limiting the yield of this crop. The most commonly used nutrients in the cultivation of sorghum is N followed by P. Normally the recommendations for the application of the N, P, and K are at rates of 190, 40, and 0 kg ha-1, respectively (Diaz de León et al, 2008), but sometimes farmers exceed these levels with the expectation of obtaining a higher yield.
In this study, nitrogen fertilization showed an important impact on the sugarcane aphid population, with a significant increase in the population as the dose of N to the crop increased. Similar results were observed in the first report on the influence of nitrogen fertilization on the sugarcane aphid by Lama et al. (2019). Several studies report that excessive nitrogen fertilization causes an increase in the aphid fertility rate, stimulating an excessive increase in the population (Aqueel & Leather, 2011; Rostami et al., 2012; Hosseini et al., 2015; Aziz et al., 2018; Dong et al., 2018). According to Bala et al. (2018), excessive doses of N result in green, succulent plants that attract pest populations. Plants with excessive nitrogen fertilization increase their dry weight, leaf area as well as their leaf chlorophyll content and grain yield. Together with the above, the increase in N dosage promotes the accumulation of proteins, free amino acids, and sugars that can attract insects. Contrasting results were obtained in the present study, where the content of total soluble proteins and reducing sugars showed a positive correlation with the sugarcane aphid population; these metabolites, in turn, had an increase directly proportional to that observed in the dose of N. Behmer (2009) proposes that the balance of nutrients in the diets of the animals is the basis of most parts of their physical condition, and he calls this the "geometrical framework". This theory assumes that animals eat an optimal amount of food to meet their demand. Depending on the composition of the feed, this optimal amount determines their "target feed intake", and as the individual moves away from the actual intake, the more significant the fitness costs will be. This theory contrasts with the results of our study, where a lower development of low-dose N in the sugarcane aphid population was observed. This theory suggests that aphids fed until their optimal N requirements were met, but in turn had an excess intake of carbohydrates (Tabs. 2 and 3). All this excess carbohydrate requires a fitness cost for disposal.
When increasing the N dose to double the plant requirement, a slight decrease in the sugarcane aphid population is noted. Sauge et al. (2010) observe that the population of Myzus persicae remained stable at low N levels and decreased slightly at high N levels, showing a parabolic response to the N level. The most considered hypotheses for this phenomenon are toxicity due to an excessive content of metabolites in the phloem of the plant and the decrease in fertility of females due to a decrease in size due to the scarcity of nutritional resources (Honek, 1993).
Opposite results to those obtained in this study are reported by Choudhary et al. (2001) who observed a significant increase in the population of Myzus persicae in canola evasively fertilized with N. Instead, many research studies reinforce the findings obtained in our study. In a study with Aphis craccivora in ornamentals, Hosseini et al. (2015) observe that increasing nitrogen fertilization levels dramatically increased the aphid population. In a study conducted by Sinha et al. (2018), an increase in the population of the aphid Lipaphis erysimi is reported with high doses of N in mustard. Similar results are obtained by Fallahpour et al. (2015) with the same pest in canola.
In our study, P showed a significant effect on the sugarcane aphid population, with an increase in the population being observed when high doses of P were administered to the crop. In a compilation of studies on the effects of plant nutrition on the order Hemiptera, Singh and Sood (2017) find that in 50% of the cases phosphorus fertilization has a positive correlation with the insect population, either increasing it or improving some biological parameter. In the other half of cases, the effect was reversed. Supporting the results of the present study, Rashid et al. (2014) find that raising the P dose in rice markedly increases the population of Nilaparvata lugens. Similar results are reported by Dash et al. (2007) on the same pest. El-Zahi et al. (2012) find that the application of P decreases the incidence of Bemisia tabaci in cotton. Supporting these results, Sinha et al. (2018) and Pandey (2010) observe a decrease in the population of the mustard aphid at high doses of P. Facknath and Lalljee (2005) mention that high concentrations of P decrease the attractiveness of the host to insect pests through the accumulation of phenolic compounds (tannin and lignin), which act as a barrier with deterrent or direct toxic effects on insects. Bala et al. (2018) mention that an excessive phosphorus fertilization can cause an exuberant growth of plants which is more attractive for some insects. In the case of the present study, this attraction can be explained by an increase in the total proteins generated by the increase in the P dose (Tab. 2), since a positive correlation was observed between the concentration of total leaf proteins and the sugarcane aphid population (Fig. 3).
It is now well understood that K influences several physiological and biochemical processes that are relevant to the susceptibility of plants to insects (Amtmann et al., 2008). In our study, the increase of the K dose had a negative effect on the sugarcane aphid population. These results can be attributed to the fact that with high doses of K, N uptake by the plant decreased considerably, and a negative correlation with the concentration of reducing sugars was also observed (Tab. 4). Wyn-Jones and Pollard (1983) show that more than 60 enzymes depend on K activity, and many of them are involved in sugar and nitrogen metabolism. Low doses of K in crops result in an accumulation of soluble sugars, especially leaf reducers (Huber, 1984; Bednarz & Oosterhuis, 1999; Pettigrew, 1999). Potassium also plays the role of osmoregulator and long-distance transport of metabolites, being indispensable for the mobilization of primary metabolites in the plant (Amtmann et al., 2008), which may explain the accumulation of proteins in the leaves in our study.
Recent studies reinforce the strong influence of K on the development of herbivorous insects. Sinha et al. (2018) report that potassium fertilization reduces the attack of the mustard aphid Lipaphis erysimi on the crop. According to results obtained by Venter et al. (2014), the application of potassium to the wheat crop can induce a certain degree of tolerance to the attack of the Russian wheat aphid (Diuraphis noxia).
Conclusions
The results obtained in our study clearly showed that fertilization with N, P and K generated remarkable effects on the main nutritional parameters of sorghum and these, in turn, on the susceptibility of the crop to attack by M. sacchari/ sorghi. The most notorious effect was observed in the total soluble proteins, reducing sugars, and concentrations of N and K in the leaf. Understanding the complex interactions that exist between the plant, the insect and the nutrients is the key to including crop nutrition in the management plan. The results obtained in this study can be used as a tactic to be considered for an integrated management of the pest in the sorghum crop.