Introduction
Environmental imbalances generated by deforestation and the increase in greenhouse gases alter crop growth and productivity significantly affecting global food security (Dhankher & Foyer, 2018). Traditional cultivation methods of crops will no longer provide optimal growing conditions, and they will increase production costs (Fischer & Melgarejo, 2020). The climatic changes that are taking place at different altitudes because of increases in temperature and radiation require additional research in order to understand the new growth conditions that will modify the phenological phases, maturation, quality and performance of crops (Ramírez & Kallarackal, 2015; Fischer et al., 2016; Ramírez & Kallarackal, 2018a). The physiological behavior of plants and the quality of their fruits depend on the characteristics of each species and variety as well as environmental conditions and crop management (Fischer et al., 2018; Fischer et al., 2020).
Therefore, studying plant ecophysiology is of utmost importance for knowing how abiotic and biotic factors of the environment affect the physiology of cultivated plants (Fischer et al., 2016) and how physiological mechanisms interact with these physicochemical and biotic factors (Lambers et al., 2008).
The feijoa (Acca sellowiana [O. Berg] Burret) belongs to the Mirtaceae family, known for its botanical richness and represented by some 121 genera with 5800 species, many of these with a high agro-industrial potential for their aromatic fruits (Farias et al., 2020). The characteristics of feijoa are similar to those of the guava (Psidium guajava) and the bayberry (Mircia acuminata), but with a different phenotype (Perea-Dallos et al., 2010; Fischer & Melgarejo, 2021).
The feijoa is native to South America, in the southern zone of Brazil and Uruguay, where it is known as "goiabeira-serrana" and "guayabo del país" (Puppo et al., 2014; Donazzolo et al., 2015). It is also known from the upper region of western Paraguay and northeast Argentina (Pachón & Quintero, 1992; Parra-Coronado & Fischer, 2013; Borsuk et al., 2015). It was introduced to other countries in the world, where it is known as feijoa or pineapple guava (Moretto et al., 2014). The most extensive production is found in Colombia, New Zealand, Georgia, Ukraine, but also in the United States, Australia, Turkey, China and with an increasing area of cultivation in Brazil (Sachet et al., 2019; Sánchez-Mora et al., 2019). This distribution in such varied countries and climates demonstrates the great potential for the plasticity that this species possesses for adaptation to very diverse environmental conditions (Donazzolo et al., 2019). Ouafaa et al. (2019) not only highlight the fruit's easy adaptation to subtropical climates but also the sweet aromatic characteristics of the feijoa fruit that favor its distribution in so many countries. However, Phan et al. (2019) state that this fruit has remained relatively unknown to many people in the world until today.
Two different populations of feijoa have been described (Ducroquet et al., 2000). One population is found in the higher altitude regions of the basalt plateau of Southeastern Brazil. This population has long and hard seeds, often with a bitter pulp and hard peel fruits, called "Brazil group" (Cabrera et al., 2018). The other population is found on the acid soils of Uruguay and the south of the Rio Grande do Sul state in Brazil. These fruits (called "Uruguay group") are sweet and contain small, soft seeds that have been distributed in many countries (Schotsmans et al., 2011). Ducroquet et al. (2000), describing these two groups and zones where the feijoas were found, mentioned that these were sites with a medium temperature of 16°C. However, the researchers also affirmed that they had no information of naturally occurring feijoas in Paraguay which were mentioned by other authors (e. g. Pachón & Quintero, 1992).
Feijoa germplasm banks are mainly concentrated in three centers: 1) the Feijoa Active Germplasm Bank (BAG) in São Joaquim-SC (Brazil), with 313 accessions, 2) the Feijoa National Center (CENAF) in La Vega (Cundinamarca, Colombia), with some 1500 accessions, and 3) the Nikitsky Botanical Garden (Crimean peninsula) that has 400 accessions (Sánchez-Mora et al., 2019). In Colombia, Parra-Coronado et al. (2016) reported commercial varieties such as clone 41 (Quimba), clone 8-4, Mammouth, Apollo, Gemini, Triumph, Rionegro, Tibasosa and some others.
In tropical areas, the tree can produce throughout the year, while in subtropical areas, production predominates at a unique time of year (Quintero, 2012). In Colombia, feijoa is a very promising fruit due to its adaptation to areas between 1800 and 2700 m a.s.l. (Quintero, 2012). Feijoa production in Colombia reached 9290 t in the first half of 2019, surpassing fruits such as cape gooseberry and pitaya (DANE, 2020), with the most important cultivation in the Cundinamarca-Boyaca mountain valleys.
Feijoa is a subtropical shrub or small tree with a maximum height of 3.5 to 5 m. It is a long-lived perennial fruit species that can produce, in technically controlled crop systems, 20 t ha-1 or more (Fischer, 2003; Quintero, 2012). The species was previously considered to be an ornamental plant (Pachón & Quintero, 1992). In highly controlled cultivation, the feijoa grows erectly with a central leader (Omarova et al., 2020), forming between three and four levels of horizontal bent laterals (Quintero, 2012). The plant has elliptical to oval shaped leaves, bright green on the upper side and 6x4 cm whitish color on the underside (Fischer, 2003).
The hermaphrodite flowers of 3-4 cm are composed of four greenish-gray sepals and a corolla formed of four white petals. In the center, there are 60-120 stamens of red filaments with white anthers, according to the particular cultivar (Fischer, 2003; Ramírez & Kallarackal, 2017). In general, A. sellowiana has barriers to self-fertilization such as the dichogamy through protogyny and self-incompatibility (Stewart & Craig, 1987), and Finatto et al. (2011) found a late-acting self-incompatibility in this species.
In Colombian plantations, the authors of this review observed that the flowers develop mainly 1) in the leaf axils of shoots of the same year, 2) on the branches bent horizontally (up to several years), 3) on the thin and mostly hanging branches in the inner crown and 4) on stump sprouts emerging after cuttings in the lower and middle part of the crown. In a study near Bogota (Colombia), Ramírez and Kallarackal (2018b) characterize two feijoa cultivars, with six different phenological stages according to the scale of the BBCH (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie): 1) bud development, 2) leaf development, 3) stem development, 4) flower emergence, 5) flowering and 6) fruit development.
Many cultivars are self-fertile, but they also allow cross-pollination by insects or birds (Ramírez & Kallarackal, 2017). The most important ornithological agent in Colombia is the blackbird (Turdus fuscater), Turdus merula in New Zealand and Acridotheres tristis, and Turdus spp., Thraupis spp. and Tangara spp. in Brazil. All these species eat the petals, which increase their sugar content immediately before and during the opening of the flower (Duarte & Paull, 2015). This fact is very important for the control of Botrytis in petals during flowering (Quintero, 2012).
For humans, the flower petals are edible and contain, according to the variety, high concentrations of antho-cyanins, flavonoids, total phenols, increased antioxidant activity and high soluble solids content (Amarante et al., 2019). Magri et al. (2020) found that the petals attain the highest peak of polyphenols, anthocyanins and ascorbic acid in the F2 state, when the petals continue to open and the anthers and filaments have a dark red color, according to the BBCH scale (Ramírez & Kallarackal, 2018b).
The fruit is a berry, mainly ovoidal in shape and with a bright green or grayish green color, smooth to rough skin. Fruit dimensions are 3-5 cm in diameter, 4-10 cm in length, weight between 20 and 250 g and contains between 20 and 40 seeds (Fischer, 2003; Schotsmans et al., 2011). Apart from fresh, un-processed consumption (with a very sweet and aromatic flavor), fruits can be processed for juices, jellies, drinks, ice creams and yogurts (Mitra, 2010; González-García et al., 2018).
Zhu (2018) highlights the effects of the fruit on health as an important source of polyphenols, vitamin C, fiber, sugars and minerals, mainly potassium. It also has medicinal value for its antibacterial, antioxidant and antiallergic activity, with a higher content of antioxidants, such as flavonoids and ascorbic acid in the fruit skin rather than in the pulp (Phan et al., 2020).
Sachet et al. (2019) confirm that ecophysiological studies are necessary to improve the cultivation of feijoa. Furthermore, Germana and Continella (2004) clearly state that ecophysiological evaluation is important for understanding the possibilities of acclimatization and the diffusion of species, including feijoa. The objective of this review is to present the results of the literature on ecophysiological topics of the feijoa, including temperature, light, altitude, water, and wind. These data should be very useful for researchers and plant breeders as well as for fruit growers.
Ecophysiological factors and their effect on feijoa
Some research exists describing the influence of temperature, solar radiation, altitude, water (precipitation, drought) and wind factors on this crop. It should be understood that in the different areas of cultivation there is not a single climatic factor that acts alone. It is the interaction of several factors that influence the plant, causing stress conditions such as drought, waterlogging, very high and low temperatures or ultraviolet light that can be detrimental to plant performance (Mittler, 2006).
Temperature
In temperate or subtropical zones, feijoa adjusts its physiology to the seasonal temperatures. Low winter temperatures inhibit the sprouting of branches and flowers are reactivated with the increase of temperatures in spring (Fischer, 2003). In temperate zones, when soil temperatures reach 8-10°C in spring, root growth begins and continues until autumn when soil temperatures drop below 8°C (Thorp, 2008). Morley-Bunker (1999) reports that feijoa in temperate zones produce larger fruits and yields in the sites with higher temperatures compared to those with lower winter temperatures.
Flowering and ripening of the fruit can be advanced by eight weeks in warmer climates compared to cold climates (Duarte & Paull, 2015). Furthermore, climate change impacts tree phenology (Ramírez & Kallarackal, 2017). However, fruits produced in cold weather are supposedly of better flavor than those produced in hot climates (California Rare Fruit Growers, 1996). This was confirmed in a study carried out in Colombia by Parra-Coronado et al. (2015a) for feijoa fruits from clone 41 (Quimba), where they found that the fruits produced at higher altitudes (cold weather) are sweeter than those produced at low altitudes (warm weather). Barrero (1993) reports that adequate temperatures for feijoa production in Colombia are between 13 and 21°C (average 16°C).
In temperate areas some authors report that feijoa requires a certain number of chilling hours (temperatures below 7 or 7.2°C according to author) to increase flower production, and this would be deficient in areas with less than 50 chilling hours (Fischer, 2003). Sharpe et al. (1993) indicated that the feijoa needs between 100 and 200 chilling hours below 7°C to sprout. For this situation, Schotsmans et al. (2011) summarize that feijoa reacts not only to changes in temperature that characterize the season in subtropical and temperate zones, but they also arise from the changes in the pattern of rainfall and as a reaction to pruning, as in Colombia (Quintero, 2012).
According to Rom (1996), the effects of temperature on a fruit tree should not be isolated from other agroecological factors, especially water and light stress. Temperature tolerance depends on the concentration of carbohydrates stored in the tissues and the level of nutrient ions in the plant. The feijoa plant is highly susceptible to very high temperatures (>32°C), combined with low relative humidity during pollination and fruit set (Duarte & Paull, 2015). In general, Quintero-Monroy (2014) observes that high temperatures in Colombia cause damage to apical and juvenile sprouts, causing a folding of the leaves. As the authors of this review presume, the fruit development time of the feijoa will decrease due to global warming (Duarte & Paull, 2015). This can affect the fruit quality increasing the concentration of sugars and decreasing that of acids (Ubeda et al., 2020). This causes the fruit to be tasteless, without a good sugar/acid ratio; therefore, varieties that do not show this behavior should be selected (Parra-Coronado & Fischer, 2013).
Feijoa is considered a subtropical fruit tree that tolerates winter frosts. It tolerates temperatures down to -10°C that can occur in the Bogota High Plateau (Quintero-Monroy, 2014) and temperatures down to -4°C do not cause significant damage in adult plants (Pachón & Quintero, 1992). In the Bogota High Plateau, temperatures lower than -4°C for more than 1 h cause losses of flowers and juvenile re-growth (Quintero-Monroy, 2014). Therefore, the authors of this review do not recommend pruning feijoa trees for 1-2 months before the frost season to avoid new (tender) shoots. Fischer (2003) observed that frost can cause abortion of leaves and small fruits, but frost also damages the fruit during its ripening, with premature browning of the affected tissues (Schotsmans et al., 2011). In a study with five feijoa varieties, Stanley and Warrington (1984) found that trees are damaged by frost with temperatures below -3°C in the summer season and below -8°C in the winter season, and the temperature can be lethal to the plant still at 4-6°C lower than these values mentioned.
Adult plants are more resistant to low temperatures (Tocor-nal, 1988), while juveniles (<1 year) can die during longer frosts. In Tenjo (Cundinamarca, Colombia) in a frost that reached -10°C, Quintero-Monroy (2014) observed that half the 1-year-old plants lost all their leaves and resprouted only at the base of the stem, while the 2-year-old plants showed slight damage and the 10-year-old plants only had some flowers burned.
In a study carried out in Cundinamarca (Colombia), Parra-Coronado et al. (2015b) found that temperature influences the growth and quality of feijoa fruits (clone 41 'Quimba'). Thus, in San Francisco de Sales with a registered average temperature of 18.3°C (1800 m a.s.l.), fruit development advanced much faster (155 d) than in Tenjo (180 d) with a registered average temperature of 12.3°C (2580 m a.s.l.). This was similar to that observed by Fischer et al. (2007) in cape gooseberry with 66 d at 17.0°C compared to 12.5°C and 75 d in the neighboring department of Boyaca. Interestingly, Parra-Coronado et al. (2016) recorded much less growing degree days (GDD) (Eq. 1) for the fruit at 12.3°C (1972 GDD) rather than at 18.3°C (2677 GDD). This can be seen as an adaptation to these colder conditions. Also, a temperature of 18.3°C produced smaller fruits (38 g) than those that grew at 12.3°C (69 g), indicating that feijoa is rather a cold-weather crop of cool Andean highland conditions. It should not be forgotten that in this study lower temperature was accompanied by higher cumulative radiation (11082 W m-2) during the development of the fruit rather than at the lower elevation and, thus, hotter site (8918 W m-2) (Parra-Coronado et al., 2015a).
where GDD are the growing-degree days (°C) accumulated during n days of fruit development, Ti is the average daily temperature (°C) for day i and Bt is the base temperature for fruit growth (1.76°C). Ti = (Tmax + Tmin) / 2.
Parra-Coronado et al. (2015b) also studied the base temperature (Bt) in 'Quimba', that is, the minimum for the growth of the reproductive organs of feijoa, and they find that feijoa is a plant well-adapted to cold conditions. They register 3.04°C Bt for the flower bud phase before anthesis and from anthesis to fruit set, while the Bt for the phase from fruit set until its harvest is only 1.76°C, lasting 189 d from flower bud until the fruit harvest at 2651 GDD. Also, in other fruit trees from Andean areas, Bt was calculated for fruit development as low as that for cape gooseberry with 1.9°C (Salazar et al, 2008) and 0.01°C for that of curuba (banana passion fruit) (Mayorga et al, 2020). Parra-Coronado et al. (2015b) recommend that the prediction of a time course based on the growing-degree days (in this case from flower bud to fruit maturity) can be applied to schedule harvest and crop management practices related to the phenological phases of the plant.
Solar radiation
Reproductive stages like flowering, pollination, fruit set, and fruit fillings are favored in free exposure to solar radiation (≥1500 h of direct sunlight per year), since they adapt well to full luminosity, as long as there are no dry and high temperature conditions (Fischer, 2003; Fischer et al, 2020). According to Quintero-Castillo (2003), in sites where there are only 1000 h of direct sunlight/year, but with very favorable agro-ecological and crop management conditions (irrigation, fertilization, pruning, pollination), it is possible to obtain a good production. Parra-Coronado et al. (2015a) harvested 44% larger fruits at higher altitudes (2580 m a.s.l.), at which the accumulated solar radiation before harvest was 20% higher than at the lower site (1800 m a.s.l.).
Despite the fact that feijoa tolerates partial shade and at sites with <1500 h of direct sunlight/year, Duarte and Paull (2015) recommended training the tree canopy to a central leader (in a pyramid shape) to take full advantage of the light. Quintero (2012) also highlights the advantages of forming pyramidal-shaped tree crowns with three branching levels of horizontal bent primary laterals, allowing a better incidence of light on the flower buds and higher photosynthetic rates of the leaves, compared to a tree using straighter branches or a free growth like a bush. Martinez-Vega et al. (2008) harvested smaller fruits in the inner and outer crown of the basal part of the feijoa tree, where only 35 and 54% of the external solar radiation falls on a full sunny day (Fig. 1).
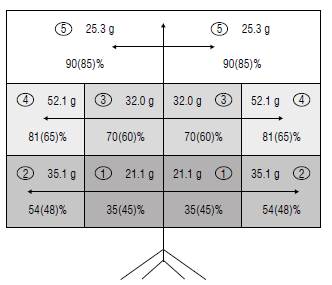
FIGURE 1 Distribution of the quadrants in the crown of the feijoa tree: 1) internal base, 2) external base, 3) internal medium, 4) external medium, and 5) superior, including the fresh weight of the fruits (g) and the percentages of the incident radiation on a sunny day (on average 1920 jumol nr2 s1) and (in parentheses) on a cloudy day (on average 348 jumol nr2 s1). Modified from Martinez-Vega et al. (2008) with permission of the Revista Colombiana de Ciencias Hortícolas.
Times of high and prolonged solar radiation that commonly occur with excessive heat and drought (as during the "El Niño Phenomenon" in northern South America) can cause fruit burning when not protected by the foliage (Fischer, 2003).
Martinez-Vega et al. (2008) studied the effect of fruit position on 6-year-old feijoa trees, cv. Quimba, trained in three branching levels with horizontal bent laterals (Fig. 1), in an orchard located at 2350 m a.s.l. in the municipality of La Vega (Cundinamarca, Colombia). The physiologically ripe fruits harvested in the outer middle quadrant (Fig. 1) showed higher fresh weight, while fruit color was more intense green at the base and inside the crown. This confirms that the fruits growing in the outer middle quadrant stand out for good overall features. The fruits of the upper quadrant develop a higher maturity ratio (total soluble solids/total titratable acidity) but with a lower weight. This confirms the large effects of the differential microclimates on the tree canopy and on the quality of the fruits, taking into account that the leaves on the upper periphery of the feijoa tree have a greater number of stomata (Naizaque et al., 2014). However, trees are exposed to elevated solar radiation (especially UV) in the high tropics and must adapt to stress conditions due to very high temperatures that can cause situations of stomatal closure and photoinhibition that also limit fruit filling (Fischer & Orduz-Rodriguez, 2012). On cloudy days, Martinez-Vega et al. (2008) recorded differences in incident radiation in the different parts of the canopy that are much less pronounced than on a sunny day (Fig. 1).
Altitude
Since the tropics are areas with thermal uniformity (i.e., without seasons of temperature), thermal zones are formed with a reduction in temperature as altitude increases, in which the only contrast is between day and night. Some authors describe for the high tropical zones the daytime as summer and the nighttime as winter (Fischer & Orduz-Rodríguez, 2012). These authors define the climatological changes that occur with increasing altitude as a reduction in temperature of 0.6°C on average per 100 m, a decrease in the partial pressure of gases such as O2, CO2, N2 and water vapor as well as decreased precipitation (from 1300-1500 m a.s.l.), while UV, visible and infrared radiation, and wind increase with tropical elevation.
The adaptation of the feijoa to the tropical highland climate comes from its area of origin (Jackson & Looney, 1999) at about 1000 m a.s.l. in southern Brazil. In Colombia, commercial feijoa plantations adapt well to a range between 1800 and 2700 m a.s.l. (Parra-Coronado et al., 2019), as do deciduous fruit trees and strawberries (Fischer & Orduz-Rodríguez, 2012). The optimal range between 2100 and 2600 m a.s.l. is apparently the best for feijoa (Duarte & Paull, 2015). Quintero-Monroy (2014) mentions that plantations >2700 m a.s.l. lack economic profitability because plants grow too slowly and show inferior production and fruit quality.
For Barrero (1993), the ideal altitudinal zone for commercial feijoa production in Colombia lies between 2000 and 2400 m, while areas lower than 1800 m are not consistent for correct crop behavior. Pachón and Quintero (1992) point out that at altitudes below 1600 m (e.g. the Colombian coffee zone) the growth of the plant is limited by the Anastrepha sp. fruit fly. However, Fischer (2003) reports that the fruit fly has been found up to altitudes of 2600 m in Tibasosa (Boyaca). In general, Quintero (2012) states that the efficient control of fruit flies (the most restrictive pest of feijoa) is easier in orchards in tropical highland areas above 2000 m a.s.l. and recommends the production of organic feijoas at altitudes higher than 2500 m.
The adaptation to these relatively high altitudes favors the thick cuticle and the small size of the leaves of the feijoa (Schotsmans et al., 2011), which reduces transpiration in dry seasons and also filters UV radiation, avoiding its mutagenic effects and favoring their phytosanitary status (Fischer & Orduz-Rodríguez, 2012).
Although the cool Andean highland conditions postpone the harvest date (Mayorga et al., 2020), this results in larger and better-quality fruits compared to lower growing sites (Parra-Coronado et al., 2015a). Fischer and Orduz-Rodríguez (2012) report that apples from plantations in high altitude tropical regions (2400-2700 m a.s.l.) stand out for their juiciness, coloration, aroma, compact pulp and firm texture.
Water
Precipitation between 700 and 1200 mm/year favors the production of feijoa, and the fruit tolerates up to about 2000 mm if there is good light and low relative humidity (RH) (Duarte & Paull, 2015) of around 70% (Pachón & Quintero, 1992). Fischer (2003) points out that the pattern of rainfall during the course of the year is important for flowering and fruit set. Therefore, a season without rain favors flowering, which is still improved if there is irrigation at these stages at the beginning of the reproductive phase and during fruit filling (Fischer et al., 2012). Quintero (2012) states that in commercial plantations an adequate and regular water supply is essential for meeting the requirements of the tree at its full vegetative and reproductive growth, allowing two harvests per year in areas where a bimodal rainfall regime prevails (Duarte & Paull, 2015). Feijoa shows certain characteristics of drought tolerance due to its thick leaf and fruit cuticles. Intense and prolonged dry periods can generate leaf, flower and fruit fall (Jackson & Looney, 1999), but they can also interrupt plant development and delay fruit ripening (Fischer, 2003). In turn, Tocornal (1988) indicates that feijoa has a very fibrous and superficial root system that increases its sensitivity to water stress, so that additional irrigation during dry seasons favors production.
Germana and Continella (2004) compared feijoa ecophysi-ological behavior with that of avocado and custard apple and found that feijoa showed low water use efficiency (WUE) and high respiration rates, primarily during flowering, when the energy demand of this tree is very high. Feijoa leaves mainly showed higher transpiration rates than the other two species, due to their low stomatal resistance that causes a WUE of only 1/3 compared to avocado and custard apple (Germana & Continella, 2004).
Quintero-Monroy (2014) states that the most productive crops of feijoa are found in areas where the full flowering season coincides with the dry season, but if the flowering peak coincides with high rainfall, the flowers fall due to the incidence of Botrytis cinerea, because these organs form small concavities, in which water accumulates and causes rot. Also, elevated rainfall makes it difficult to pollinate flowers, because the avian pollinating agents do not fly much in rainy seasons to avoid wetting their plumage or the negative effects of falling water on the leaves of the plant (Quintero-Monroy, 2014). The same author points out that poorly pollinated flowers could be aborted prior to fruit set and, in this case, subsequent fruits that are formed are low-caliber and asymmetric.
Drier conditions favor floral induction in feijoa (Peña-Baracaldo & Cabezas-Gutiérrez, 2014) confirmed in plants of cv. Tibasosa (3 years old) where a deficit irrigation of only 25% for 406 d induced the highest number of flower buds compared to irrigation with 100,75, 50 and 0% of water. A dry season clearly benefits flowering in this species, while a wet one decreases it (Quintero-Monroy, 2014). However, due to climate change, there are no longer well-defined dry seasons, because dry seasons now occur at different times of the year. To have good feijoa production, pruning should be carried out about 60 d before full blooming and this should be during the dry season. But due to the uncertainty of the climate, it is possible to increase flowering with the application of phosphorus, using KH2P04 (0.5%), as found by García et al. (2008) in cultivar 41.
Water stress can limit the accumulation of dry matter (DM) in feijoa leaves. Therefore, Omarova et al. (2020) recommend selecting varieties that are more resistant to water deficiency and that do not decrease their concentration of leaf DM under the conditions of water stress. These authors registered varieties such as 'Sentiabraskaja' that, during water stress, accumulated a higher leaf DM than others, and the dry season coincides with the time of fruiting. Likewise, Omarova et al. (2020) observe that, in periods of high thermohydric stress, the studied varieties increase the content of the osmoprotector proline in leaves between 1.5 and 2.8 times. These authors also stated that the resistance of plants to adverse environmental factors is highly determined by the activation of the enzymatic system of antioxidants that can inhibit the damaging effect of oxidative stress.
In general, feijoa thrives well in regions that have a RH of around 70% (Pachón & Quintero, 1992) and RH >70% for prolonged periods increase the incidence of diseases, mainly Botrytis during flowering. Therefore, Duarte and Paull (2015) highlight dry conditions in combination with high luminosity as the most important for avoiding this disease and guaranteeing a high percentage of well-curdled fruits. Feijoa resists high RH during short periods (Fischer, 2003). In areas where these humid conditions are regularly prolonged, the formation of the cone-shaped (pyramidal) tree is the most productive, despite the incidence of Botrytis compared to trees in free growth or with an excess of branches (Quintero-Castillo, 2003). In addition, high RH favors the incidence of epiphytes on the trunk and branches of the crown as well as mosses and lichens that secrete substances affecting plant health (Quintero-Monroy, 2014).
Studies in Suba (Cundinamarca) by Galvis et al. (1999) and in Tibana (Boyaca) by Naizaque et al. (2014) show that leaf transpiration of the feijoa is greater in the upper stratum of the crown than in the lower, with more stomata in the upper periphery (91/mm2) than in the lower part (78/mm2) of the plant (Naizaque et al, 2014). These authors observe that the transpiratory rate increases according to increases in temperature and solar radiation in the crown but decreases with the increase of RH in the tree (Fig. 2).
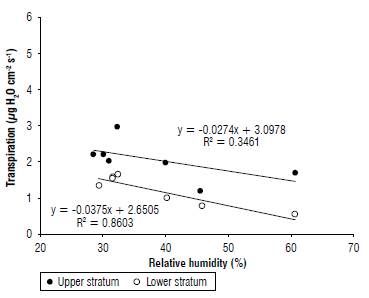
FIGURE 2 Effect of the increase in relative humidity on leaf transpiration in the upper and lower stratum of the canopy of feijoa trees (Naizaque ef al., 2014). With permission of Revista U.D.C.A Actualidad & Divulgación Científica.
The feijoa plant shows some tolerance to salinity in the soil and irrigation water, but it reduces tree growth and production (Duarte & Paull, 2015). However, Casierra-Posada and Rodriguez (2006) do not observe differences in dry mass distribution and tree production when they add 0, 20, 40, 60 and 80 mM NaCl to the substrate (corresponding to an electrical conductivity (EC) of 2, 8, 4.6, 6.1, 8.4 and 11.0 dS m-1), increasing the level weekly until reaching this concentration of salts. They only record reduced evapotranspiration with increasing salinity. The authors of this review observed leaf fall and low flowering on feijoa trees in a plot in Mosquera (Cundinamarca) with an EC between 4 and 6 dS m-1. Due to the lack of research on this topic, more studies are necessary.
Wind
Feijoa trees, due to the strong structure of branches and their small and thick leaves, are relatively resistant to the wind (including salty wind from the sea), for which they are used mixed with other species in live barriers against this element (Duarte & Paull, 2015). Gentle winds are important for drying the plant after a rain, avoiding fungal diseases, cooling the leaves on hot days, and renewing the C02 concentration in the crown (Fischer & Orduz-Rodriguez, 2012).
Conclusions
The study of environmental effects - now altered by climate change - is of utmost importance for finding feasible growing sites for promising fruit species. The feijoa plant in Colombia adapts to climatic conditions between medium and cold climate (1800-2700 m a.s.l.), but high temperatures affect the beginning of fruiting. Due to its site of origin in the highlands, the plant resists temperatures below 0°C and has a base temperature of 3.04°C for the initial phases of its reproductive stage. It is well-adapted to a great number of direct sunlight hours, but extremely high solar radiation intensities decrease the size and can burn the fruit. The greater radiation accumulated during fruit development produces larger fruits. For floral induction, flowering and fruit set, drier environmental conditions and a relative humidity around 70% are favorable. There exists no concluding information on the tolerance to salinity and waterlogging. The strong trunk and branch structure of feijoa favors its good wind resistance.















