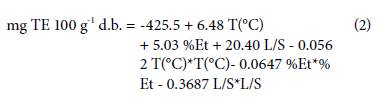Introduction
Quinoa is an annual plant from the Andean region of South America, cultivated from Colombia to Argentina and Chile. In recent years, there has been a progressive increase in quinoa crops, especially in Bolivia, Peru, and Ecuador, which have been the main producers (FAO-ALADI, 2014).
Quinoa exhibits multiple abilities to adapt itself to solar radiation, temperature, water availability, and atmospheric CO2 concentration, allowing its cultivation in different agroecological zones (Zurita-Silva et al., 2014; Meló, 2016; Reguera et al., 2018). It is a plant with additional notable agronomic adaptations to different adverse weather conditions like drought, high salinity, and frosts (Ruiz et al., 2014; Ruiz et al., 2016).
Quinoa is considered a pseudocereal because of its particular composition, making it a grain of special interest as a human food. Its grains are exceedingly nutritious (López et al., 2011; Padrón Pereira et al., 2015). Quinoa seeds are rich in lipids, carbohydrates, polyphenols, fiber, and proteins (Padron-Pereira et al., 2015; Tang et al., 2015; Fischer et al., 2017; Vidaurre-Ruiz et al., 2017; Jaikishun et al., 2019). The protein contents range between 12% and 16% depending on the quinoa cultivar. They have high nutritional value because of the presence of essential amino acids such as leucine and isoleucine. These seeds contain proteins without gluten, and they are currently appearing in diets free of this component (Alvarez-Jubete et al., 2009).
Argentina has great diversity of foods that protect biomolecules from oxidative damage by different mechanisms. There are two fundamental strategies to protect the organism from free radicals: the enzymatic and non-enzymatic (endogenous and exogenous) (Wu, 2015). The endogenous strategy requires external support, but exogenous antioxidants are recommended as they can capture free radicals from oxygen and chelate transition metals. Exogenous antioxidants can generate reactive oxygen species (ROS) by the Fenton/Haber-Weiss reaction in its free ionic state. This phytochemical benefit is present in quinoa, but it varies in different cultivars of the same plant (Tang et al., 2015).
In addition to being incorporated into the daily diet, concentrate extracts can be considered as possible sources of antioxidant compounds that can be used for the enrichment of other products with antioxidant compounds. The extraction process is regulated by different variables like extraction temperature, the nature of the extraction solvent, and its concentration, treatment time, and the state of aggregation of the substrate. Engineering aims to develop different optimal processes to meet the variable values and costs.
In this research, the optimal values of the pretreatment parameters for the extraction of antioxidant compounds were studied. These were the drying temperature of quinoa grains, liquid/solid ratio, and the ethanol concentration in the extraction solvent.
Materials and methods
Quinoa was provided by the Quinoa Real company, whose crops are located in Yavi, Jujuy, Argentina (22°7'47" S, 65°27'44" W). The cultivar used was Real Blanca. Quinoa was preserved in glass flasks of 5 L with hermetic covers and stored in the dark until used. The trials were carried out in a uniform batch material.
Obtaining antioxidant compounds
Following the wet de-saponification treatment of quinoa, the grains were dried in a forced convection laboratory dryer (Tecnodalvo Model CHC/F/I, Argentina) until reaching a moisture content of 12%. This process was required to avoid the deterioration caused by microorganisms. De-saponified and dried quinoa grains were ground in a mill (IKA, Germany) to pass through a 40-mesh sieve.
The flour obtained was added to a mixture of water and ethanol for 1 h in an orbital shaker (OS-20 Orbital Shaker, Boeco Germany, Germany) at 25°C. This solution was filtered and conserved at 4 ± 1°C in a caramel-colored container, until subsequent tests. Water-ethanol mixtures were used as extractants because of their efficiency, selectivity, low cost, low toxicity, and ease of removal.
Antioxidant capacity determination
The compound 2,2-Diphenyl-1-picrylhydrazyl (DPPH) is a stable radical that has an intense violet color and absorbs at 517 nm. The method proposed by Brand-Williams et al. (1995) evaluates the antioxidant capacity by measuring the reduction of the absorbance. It translates into a DPPH concentration decrease because of the scavenging effect.
One ml of the quinoa water-ethanol solution was reacted with 5 ml of 100 DPPH solution prepared at the moment of use. The mixture was conserved at room temperature in the dark for 50 min. Then, absorbance at 517 nm was recorded by using a UV-1800 spectrophotometer (UV-1800 Spectrophotometer, Shimadzu, Japan). The results were expressed as Equivalent Trolox (ET) 100 g-1 quinoa on dry bases (d.b.). A calibration curve was obtained using a Trolox standard solution.
The linear equation was as follows:
with R2 = 0.996, where y = mg de ET/L of solution and x = absorbance.
Experimental design and statistical analysis
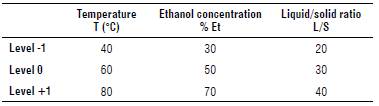
The optimization of the extraction process of compounds with antioxidant capacity was performed based on an experimental design of three variables and three levels; therefore, a face-centered central composite design was used. The parameters and their levels were as follow: grain drying temperature (T) of 40°C, 60°C and 80°C, liquid/ solid extractant ratio (L/S): 20:1, 30:1 and 40:1, and ethanol concentration in the solvent (% Et): 30%, 50%, and 70% v/v.
Table 1 shows codified values of the levels (variables) used in the experimental design and their corresponding real values for the entry factors.
Eighteen experiences in a group of 8 were required to check the two-level design (-1, 1) to the three factors (2k, k = 3); Four replicates were used on the central point to evaluate the pure error, and the remaining six trials to test faced-centered values (star points).
Linear interaction and quadratic coefficients were evaluated by an ANOVA (P<0.05). F-test and probability (p) were determined to analyze the significant statistical contribution of all the terms. The results of the experimental design were processed by applying multiple regression analyses. The model's goodness of fit was checked by the coefficient of determination (R2) and the significance of the model terms was established with a confidence level of 95%. The process conditions were optimized by response-surface graphics. All the analyses were performed using the free version of the Minitab 18 software (Minitab, Pennsylvania, USA).
Results and discussion
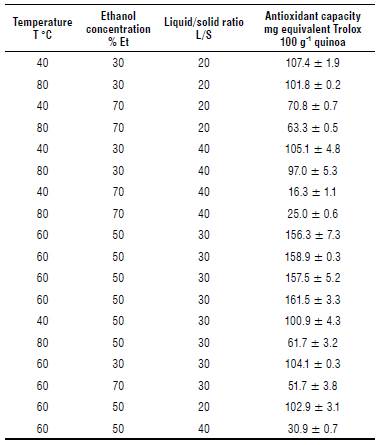
The values of the antioxidant capacity obtained for the different test conditions are shown in Table 2.
The antioxidant capacity values were coincident with the range published by Vollmannova et al. (2013), who evaluates the antioxidant capacity of five cultivars of quinoa by the DPPH method. They report values between 65.08 mg and 310.36 mg ET 100 g-1 quinoa. Valencia et al. (2017), using 24 Peruvian quinoa accessions by the DPPH method achieve values between 121.66 mg and 299.28 mg ET 100 g-1 of quinoa. It would be possible that the antioxidant capacity differs between plant varieties and among the parts of the same plant and the maturation phase of each plant organ (Sawa et al., 1999). Factors such as soil characteristics, climatic conditions, and storage conditions could modify the content of antioxidant capacity compounds (Naczk & Shahidi, 2006).
A quadratic equation (Eq. 2) was obtained by the software. It showed the effects of each factor, and its interactions according to the antioxidant capacity.
The statistical analysis (Tab. 3) showed that the percentage of ethanol and the L/S ratio had a significant effect (P<0.05) on the antioxidant capacity as well as the quadratic interaction of temperature, percentage of ethanol, and the L/S ratio. A second-degree polynomial model could be used to represent the relationship between the selected parameters. The percentage of ethanol in the solvent showed the most influence.
TABLE 3 Antioxidant capacity values of extracts of quinoa seeds for different conditions of drying temperature (°C), percentage of ethanol (% Et) and L/S ratio.

Figure 1 shows the behavior of the antioxidant capacity according to the three studied variables. When setting the L/S ratio (Fig. 1A), the antioxidant capacity increased at temperature values near 60°C. Maintaining a constant temperature, the antioxidant capacity was the maximum when alcohol concentration in the solvent was closed to 40% (Fig. 1B). Setting the ethanol concentration (Fig. 1C), the highest antioxidant capacity value was obtained when the L/S ratio was near 30:1, and it was lower for 20:1 and 40:1.
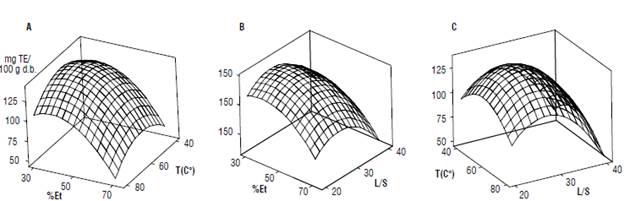
FIGURE 1 Antioxidant capacity response surface, expressed as mg Trolox equivalent of 100 g-1 of quinoa, according to the process variables: drying grain temperature (T), % of ethanol, and liquid/solid ratio (L/S). A) Setting values of the L/S ratio = 30:1; B) Setting values of T = 60°C; C) Setting values of % Et = 50 % v/v.
The values of process variables that maximize the antioxidant capacity response were 58°C grain drying temperature, 39% v/v ethanol concentration in the extractant, and a L/S ratio of 28:1.
Miranda et al. (2010) evaluate the impact of different drying temperatures on nutritional properties, the content of total phenolic compounds, and the antioxidant capacity of quinoa grains. They find that drying temperatures between 60°C and 80°C lead to degradation of total phenolic compounds. Vidaurre-Ruiz et al. (2017) find that phenolic compounds, flavonoids and betalamic pigments of Negra Collana, and Pasankalla varieties of quinoa decrease significantly after the drying process, while other compounds with higher antioxidant capacity are formed.
The optimum L/S ratio used in the extraction will depend on two basic considerations: the maximum content of the solvent is limited in practice by the consequences of the dilution of the substrates of interest and the performance of the extract according to the compounds of interest is directly related to the content of the solid from the starting material. The optimum value will result from the implementation of these considerations (Carciochi, 2014).
Regarding the solvent, the increase of the antioxidant capacity for extractant mixtures with 39% of ethanol indicates a higher presence of bioactive compounds of hydrophilic nature in quinoa (Repo-Carrasco-Valencia & Serna 2011; Stikic et al, 2012; Abderrahim et al, 2015; Fischer et al, 2017; Valencia et al, 2017). Since we propose that the extractant can be used in the food industry, the use of non-toxic solvents is crucial. Particularly, water-ethanol mixtures are most commonly used to obtain antioxidant extracts, due to their efficiency, selectivity, low cost, low toxicity and easy removal (Silva et al, 2007; Wang et al, 2008; Zhang et al, 2011; Gong et al, 2012). By changing the proportion of water and ethanol, solvent polarities change, modifying the nature of the extract, according to the different solubility of phenolic compounds present in the original matrix (Galvan d'Alessandro et al, 2012).
Conclusions
In this preliminary study, optimal values of grain drying temperature, ethanol percentage in the extractant mixture, and the L/S ratio to the extraction process of antioxidant compounds from quinoa were established (Chenopodium quinoa Willd.). The optimal processing values were a L/S ratio of 28:1, 58°C and 39% v/v of ethanol in the extraction solvent. The ethanol concentration was the most influential variable in the extraction of the antioxidant compound. Further research and a consideration of other processing variables, such as temperature and time of extraction, need to be carried out.