Introduction
Soybean (Glycine max, Fabaceae) is a relevant protein source (~16 g proteins/100 g of soybean, or 36-56% dry weight of soybean) that contains all the indispensable amino acids for humans; therefore, it is comparable to chicken or eggs. Soybean seeds contain around 20% lipids and are used as a source of protein in the diet of animals, such as poultry, pigs, or cattle. Their uses in human food include cooked seeds as part of sauces and potages, soy milk, soy flour for cakes, cookies and other baked goods. Soybean is a substitute for meat in vegetarian foods and lecithin is also extracted from its oil. At industrial plants, it is used in the manufacture of metalworking fluids, plastics, surfactants, solvents, and disinfectants. Other parts of the plant are also used as animal feed or green manures (Lusas & Riaz, 1995; Mateos-Aparicio et al., 2008; United Soybean Board, 2017).
The plant possesses sugars, insoluble and soluble fiber, vitamins, and minerals. The primary use of soybean meal is as animal feed (~98%) or as industrial substrate (Widholm et al., 2010). Genetically modified (GM) soybean is the most important transgenic crop representing ~91 million ha (50% of global area). A world production of soybean of 349 million t was registered in 2019 (FAO, n.d.). GM soybean represents 75% of the global soybean cultivation. In 2019, The United States (the first producer of GM soybean since 1996) produced 96 million t (31 million ha), and Brazil produced 114 million t (31 million ha) (Lee et al., 2013; FAO, n.d.; ISAAA, n.d.) (Tab. 1).
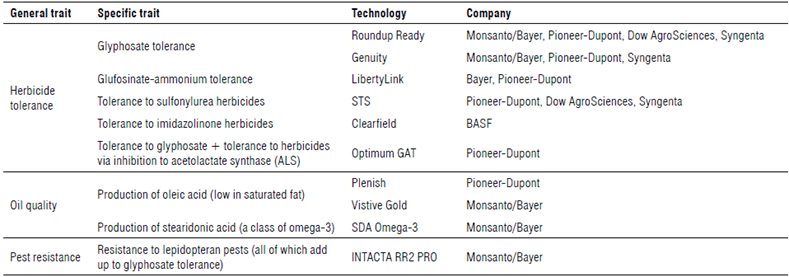
TABLE 1 Brief overview of GM technologies in commercial soybean. Thirty-nine events have been developed for herbicide tolerance, oil quality and insect resistance according to ISAAA (n.d.).
Glyphosate (N-(phosphonomethyl) glycine, (C3H8NO5P)) is a systemic phosphonate herbicide. It is a crystalline powder with a density of 1.704 g cm-3, molecular mass of 169.1 g mol-1, and solubility in water of 1.01 g/100 ml (20°C) that decomposes at 187°C. Glyphosate is an aminophosphonic equivalent of the glycine that acts as a systemic herbicide absorbed through plant leaves. This herbicide is used on commercial crops to control weeds and has been available in the market since 1974 as Roundup®. Glyphosate blocks the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) in plant cells responsible for triggering the synthesis of aromatic acids (phenylalanine, tyrosine, and tryptophan), catalyzing the reaction of shikimate-3-phosphate and phosphoenolpyruvate to form 5-enolpyruvyl-shikimate-3-phosphate as product. Since 1996, GM soybean expressing glyphosate tolerance has been marketed in the United States (Duke & Powles, 2008; Duke & Cerdeira, 2010).
Agrobacterium tumefaciens is a plant pathogen that is widely used on GM crops, as it can transfer a fragment of its 200 kb tumor-inducing (Ti) plasmid (T-DNA) as a vector of specific transgenes that is expressed in the plant (Bourras et al., 2015). Soybean is transformed via organogenic and embryogenic methods (e.g., protoplast, cell, tissue, and organ culture, and subsequent regeneration of plants). An efficient regeneration and Agrobacterium-based gene transfer procedure established on cotyledonary node explants is applied in soybean (Paz et al., 2004; Jamsheed et al, 2013; Lee et al, 2013).
The commercial use of GM soybean is limited by patents protecting each element used in a biotech process. This process involves DNA sequences comprising coding regions (e.g., event MON87751 that contains specific sequences for insect resistance), regulatory regions (e.g., event dp-305423-1 that possesses an acetolactate synthase gene conferring tolerance to applications of the herbicide sulfonylurea), vectors (e.g., patent KR102054567B1 that describes the recombinant clone vector DBN01-T), bacterial strains (e.g., patent US20200385746A1 that protects Rhizobium-mediated transformation in soybean), or tissue culture protocols (e.g., patent AU691423B2 that protects a protocol to regenerate soybean plants from cotyledonary nodes). This leads to unfortunate decisions that directly influence scientific developments. Two specific cases can be cited: i) the North American Strawberry Growers Association decided to suspend research focused on the development of GM strawberry with resistance to fungus because of patent complexity; ii) the University of Michigan was forced by a judicial decision to destroy GM lines of turfgrass due to the legal action between two companies for patents related to a coding gene and a promoter used in the approach (Thomas, 2005).
Golden rice is a GM crop that produces beta carotene, a precursor of vitamin A. There are advantages for the Golden rice project such as health benefits and low-cost release in emerging nations. Currently, Golden rice is far from being released to developing countries, as there are 40 patents involved in the project that represent a negative factor in the adoption of this technology (Kowalski et al., 2002). For Colombia, 59 patents are involved in the possible development of a GM rice line containing a cry1Ac gene (Diazgranados et al., 2016). Five corporations possess a great number of patents of GM crops. The following are a few examples of the events by corporation (the complete list of the GM events is available in the GM approval database (ISAAA, n.d.)): Monsanto/Bayer (alfalfa KK179 x J101, canola GT200 (RT200), cotton MON1076, corn GA21, potato BT10, soybean MON87705, tomato FLAVR SAVR, wheat MON71800); Dupont (canola 73496, cotton 19-51a, corn 4114, soybean DP305423); Syngenta (cotton COT102 (IR102), corn 3272); Bayer (canola HCN92 (Topas 19/2), cotton GHB614, corn T14, rice LLRICE06, soybean A2704-21, sugar beet T120-7), and Dow Agrosciences (cotton 281-24-236, corn DAS40278, soybean DAS68416-4) (Wright & Pardey, 2006) including exclusive cross-licenses (Pisano, 2006). Monsanto was purchased by Bayer in 2016, and Syngenta was acquired by ChemChina in 2017. If an independent GM seed producer wants to generate and/ or commercialize GM varieties, the seed company must consider licensing charges. However, intellectual property rights (IPR) of the elements used in a technology do not necessarily prevent its commercial use. Patents are limited to national jurisdiction and to a specific time (20 years) accepting exceptions according to the local regulation (Baker, 2019). By the end of this period, the patent enters the public domain which means that it is open for everyone to use. It is known that once a medical corporation, or similar organization, generates a new pharmacological compound to be used for a disease, it is covered under a patent contract conferred for around 20 years. When the patent time has run out, the substance can be manufactured and marketed by other companies and the pharmacological compound is termed as "generic". This also applies when the company that owned the patents declares them to be inapplicable, invalid, or abandoned, or the market region for the drug has no patent protection application. As a result, the control of the patent is removed, causing a considerable drop in costs that may be more acceptable to society (Grushkin, 2012). Therefore, it is very important to develop a well-documented and specific freedom to operate (FTO) analysis for a GM technology and a specific country (Hincapié Rojas & Chaparro-Giraldo, 2013; Lamprea Bermúdez & Salazar López, 2013). This article provides a step-by-step guide on how to develop a particular glyphosate-tolerant GM soybean event, based on experience gathered from the application of revised protocols supported by the literature, the use by public institutions, and patents related to transformation. An epsps gene and A. tumefaciens were used in an FTO basis to evaluate whether the GM soybean associate IPRs have expired or are about to expire, and to determine if they have been settled in the region of interest.
Freedom to operate study
Farmers from the United States and Argentina began to plant GM soybean around 1996 after it started to be marketed under the Roundup Ready trademark in the USA. GM soybean occupies around 95.9 million ha in the world (almost 50% of the GM crops worldwide). In 2018, the GM soybean area planted in the USA was 34.08 million ha. Brazil occupied the second position in the world with a production of ~34.86 million ha. GM soybean cultivation has expanded throughout Argentina with 18 million ha in 2018. Monsanto (now Bayer) was not capable of achieving patent protection for GM soybean with tolerance for glyphosate (Roundup Ready soybean seed) in Argentina. The company had requested the IPRs it held in Europe, but the Supreme Court of Argentina rejected that request based on the national patent law that regulates the patent duration of 20 years. Monsanto has registered some GM soybean events related to glyphosate tolerance in Argentina, (e.g., MON87701 x MON89788 and MON89788), perhaps using plant breeder rights (Brookes & Barfoot, 2018; Fries et al., 2019; ISAAA, n.d.).
An FTO study that considers the importance of generating GM soybean with marketing possibilities, before the laboratory activities and at the start of the biotechnological process, should be developed to avoid, as far as possible, the violation of third-party rights. Initially, a list of elements (materials and protocols) to be used in the development of a GM line must be in place. Then, a specific country search must be carried out based on the requested patents/current patents, including plant breeder's rights or "plant patents" (regarding the plant varieties) for each element. Data can be recovered from the United States patent office database (USPTO) (https://www.uspto.gov/patents/search), the European patent office database (ESPACENET) (https://worldwide.espacenet.com/), the Lens suite (https://www.lens.org/lens/), PATENTSCOPE from the World Intellectual Property Organization (https://patentscope.wipo.int/search/es/search.jsf), and Google Patents (https://patents.google.com/). In each of these websites, a regional patent database needs to be requested during the planning stage. Regional patent databases should be studied. Also, biological specialized databases can be reviewed, such as Nucleotide from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/nucleotide/), or Phytozome (https://phytozome-next.jgi.doe.gov/), to determine the origin of the selective biological information (Wyse & Luria, 2021). Data about patents related to glyphosate tolerant soybean should be obtained during the period from 1996 to the present day (Jefferson, Graff, et al., 2015; Goforth, 2017). Therefore, there is a growing need for competent lawyers specialized on GM intellectual property at this stage.
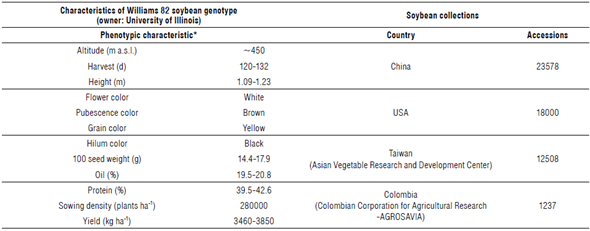
Patent data should focus especially on claims and year of application without forgetting the importance of the overall description of the invention. An FTO analysis involves the search of patents and their claims because they allow an understanding of the set of approaches that explain the developmental process of a GM crop: cloning vectors, DNA sequences, Agrobacterium strains, methods, transfer material, or confidential agreements (Chi-Ham et al., 2012; Miralpeix et al., 2014; Zanga et al., 2015). At the same time, an FTO analysis provides a parallel strategy to develop the same product in a structured way with a focus on commercialization without requirements for additional payments from licenses (Sommer, 2012). A GM soybean project based on FTO requires legal assistance from a lawyer, at least in its first instance of design. One of the first things that must be identified to achieve the goal of a GM soybean event is the soybean variety. Some countries have patent systems that prevent patent protection of certain products, such as germplasm for research and breeding (Correa, 2014). Likewise, if native genetic material is relevant in a biotechno-logical project, a contractual instrument for the appropriate access and utilization of biological and genetic resources respectful of traditional knowledge may be necessary (Deplazes-Zemp, 2019; Heinrich & Hesketh, 2019). A large number of countries have achieved significant progress in research and development on new soybean varieties in the light of local conditions (Tab. 2) (Cober et al., 2009). Just in Africa, there are 13 countries developing local soybean varieties (Benin, Cameroon, Ethiopia, Ghana, Kenya, Mali, Mozambique, Nigeria, Rwanda, Sudan, Uganda, Zambia, and Zimbabwe) (Santos, 2019). There is a program that involves the development of a genetic improvement of soybean for the tropical Colombian environment since 1960 (Tab. 2) (S. Caicedo, personal communication, 12th May 2017). Therefore, it is best to have a local soybean variety adapted to the environmental conditions of the region of interest, if possible.
TABLE 2 Standard soybean genotype collections. Transgenic processes can be influenced by virulence of Agrobacterium strains, plant species or plant varieties with predisposition to being infected (genetic background), and the design of expression cassettes.
*Soybean genotypes adapted to various thermal zones (≥24°C, humidity ~80%), with productivity of ~3500 kg ha-1, a vegetative period of -100 d, and highly sensitive to the photoperiod.
Our experience in the FTO analysis of GM soybean shows that patent application protects the following: i) DNA promoters or termination regions (CaMV35S, Gmubi, Nos) including their use in vectors for genetic transformation of mono- and dicotyledonous plants; ii) epsps gene and its variants and possible uses for genetic transformation of plants; iii) transit peptide (e.g., Petunia hybrid); iv) vectors (pCAMBIA vector); v) transformation and regeneration methods, and vi) plant varieties through a special protection known as "breeder's right" (1978 Act and 1991 Act on Common Provisions for the Protection of the Rights of Breeders of Plant Varieties - International Union for the Protection of New Varieties of Plants - UPOV) (Brenner, 1998; Dattée, 2009). According to international guidelines, a natural gene sequence cannot be patented; however, in some countries, patenting a gene sequence isolated from the environment is allowed if it is cloned in a vector (Jefferson, Köllhofer, et al., 2015). The pCAMBIA series is a set of protected plant molecular biology vectors with complete access for research and used for developing new varieties of plants (Jefferson, 2008).
A difficulty in this respect is that several methods are covered by patents that sometimes are not taken into consideration for the development of GM soybean for glyphosate tolerance. On some occasions, it is said that these techniques are being used by the public sector without giving further details. The following section describes our specific methods used when processing GM soybean for glyphosate tolerance and some frequently related patents.
Laboratory procedures and IPR
This section provides a technical guide framework relevant to the design and development of soybean transformation for glyphosate tolerance, based on our experience and perception of IPRs trends. According to Nottenburg and Roa Rodríguez (2008), those elements that have been patented are transformation vectors, vector genes, transgenes, vector design, methods for making recombinant Agrobacterium with an engineered vector, recombinant Agrobacterium incorporating engineered vectors, improved Agrobacterium strains for transformation, methods of preparing plant tissue for transformation, methods of transforming specific plants, and transformed plants and plant cells.
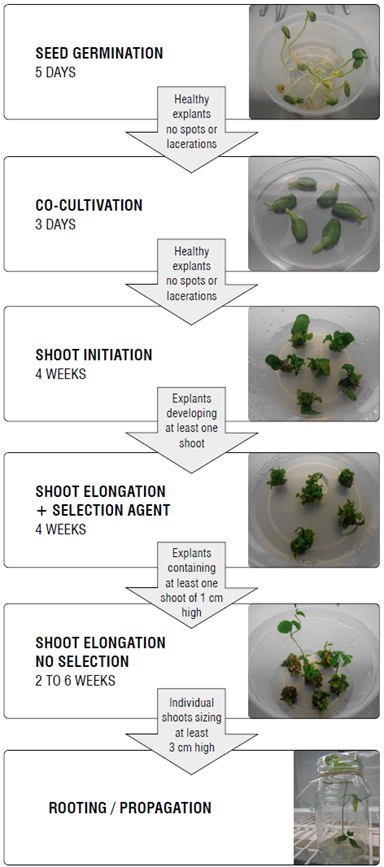
First, the available soybean seeds must be germinated and regenerated. Soybean seeds must be surface sterilized for 16 h using chlorine gas (4.1 ml of 10 N HCl, 100 ml 5% NaClO). Sterilized seeds are germinated in 0.7% agar medium, pH 5.8, for 5 d (27°C, 16/8 h photoperiod). Then, seed regeneration is monitored using a culture medium containing 1X Gamborg vitamins, 1X B5 salts, 30 g L-1 sucrose, 1.67 mg L-1 BAP, 3 mM MES, and 0.7% agar at pH 5.7. Regeneration containers are incubated for a 16/8 h photoperiod at 27°C. Five days later, the number of germinating seedlings must be determined. Regeneration is continuously monitored for four weeks. Regeneration rates are calculated based on the number of explants with shoots and the number of shoots by explant. Some patents cover similar but non-identical protocols enabling seed regeneration (e.g., EP1517991A4 (application June 22, 2002, and withdrawn status) and US5824877A (application July 22, 1988, and expired status)).
The next step is to design an expression cassette that must be introduced into a transformation vector. We prefer pCAMBIA vectors whose principal characteristics involve high copy numbers, 35S promoter, kanamycin or hygromycin B as selection markers, and GUS as screening marker. Also, pCAMBIA vectors have an FTO with availability for academic research without charges, and licenses for profit companies (for more details please visit https://cambia.org/). Ideally, an expression cassette should be designed in such a way that all the elements are stable with the help of molecular biology tools (PCR, enzyme digestion, cloning and ligation, etc.). This expression cassette can also be designed with an aggregation of genetic elements on a modularized principle through bioinformatics for chemical synthesis. When the expression cassette is obtained from a specialized company, it is important for the agreement to have an unrestricted right of utilization, and to avoid clauses as "only for research". The expression cassette includes a promoter, a sequence of chloroplast transit peptide (e.g., CTP from petunia), a CP4-epsps gene (from Agrobacterium sp. strain CP4), and a terminator sequence. Expression of transgenes is a random process caused by the codon usage or RNA regulation. According to the plant codon usage associated with transcriptional regulation and translational efficiency, mRNA stability, splicing at the mRNA level, use of special promoters, removal of polyadenylation signals and cryptic splice sites, and elimination of any potential RNA secondary structure adjacent to the translational start codon, the use of modified prokaryotic genes has allowed reaching levels of expression up to 100 times greater than transgenes without modifications (Jackson et al., 2014). A search of patent databases shows that the patents covering the CP4-epsps gene expired in 2014.
Once the designed expression cassette is synthetized according to the instructions given in the preceding paragraph, it must be introduced into A. tumefaciens. The recombinant strain can be maintained on a Luria-Bertani (LB) medium (50 mg L-1 kanamycin). A hypervirulent strain of A. tumefaciens, ATCC53213, carrying a pMON546 vector with a petunia epsps gene has been described by Kishore and Shah (1988) and Shah et al. (1986) in the patent US4971908A (application April 22, 1988, and expired status) and the patent US4940835A (application July 7,1986, and expired status), respectively. Agrobacterium strains must be characterized using PCR: i) Ach5FtsZ primers (F: 5'-GAACTTACAGGCGGGCTGGGT-3', R: 5'-CGC-CGTCTTCAGGGCACTTTCA-3', product: 369 pb) are specific to A. tumefaciens LBA4404; ii) C58GlyA primers (F: 5'-CCACCACCACGACGCACAAGTCT-3', R: 5'- TGC- CGAGACGGACACCCGAC-3', product: 423 pb) are useful for the detection of C58C1, EHA101, EHA105, and GV3101 strains; iii) pTiBo542 primers (5'- CCCGCTGAGAAT- GACGCCAA-3', R: 5'- CCTGCGACACATCGTTGCT- GA-3', product: 766 pb) are specific to EHA101 and EHA105 (differentiated from C58C1, LBA4404 and GV3101 strains), and iv) nptI primers (F: 5'- CTGCGATTCCGACTCGTC-CA -3', R: 5'- CGGGCAATCAGGTGCGACA-3', product: 572 pb) are special for EHA101 only (Deeba et al., 2014). In our experience, A. tumefaciens strains EHA101 and EHA105 are useful for soybean transformation, probably because of their virulence. We tested another bacterium, Sinorhizobium meliloti (see https://cambia.org/), to see if we could recreate the soybean infection; however, the results were not encouraging. Agrobacterium cultures (OD650 = 0.6-1.0 at 28°C, 250 rpm) must be used for infection of explants (Guo et al., 2020). A bacterial pellet obtained by culture spinning (8000 rpm, 4 min) must be resuspended in cocultivation media (1X Gamborg vitamins, 0.1X B5 salts, 1.67 mg L-1 BAP, 0.25 mg L-1 GA3, 3% sucrose, 20 mM MES, 200 μM acetosyringone, pH 5.7).
Cotyledons must be excised at the junction between the hypocotyl and the half-way point of the cotyledon, five mm below the cotyledonary node, and a cut is performed to divide the cotyledonary explants. The plumule is eliminated and two small incisions are made on the cotyledonary node. Explants are infected with recombinant A. tumefaciens (containing a designed expression cassette as described above) in coculture broth (30 min), and then inoculated on coculture solid medium (1X Gamborg vitamins, 0.1X B5 salts, 1.67 mg L-1 BAP, 0.25 mg L-1 GA3, 3% sucrose, 3.9 g L-1 MES, 200 acetosyringone, 0.7% agar, and pH 5.7) with the adaxial side down. The co-cultivation plates are incubated in the dark for 3 d at 28°C. The explants are then rinsed in sterile water twice. After the last rinse (using 350 mg L-1 cefotaxime), explants are transferred into shoot induction medium (1X Gamborg vitamins, 1X B5 salts, 30 g L-1 sucrose, 1.67 mg L-1 BAP, 3 mM MES, 0.7% agar, pH 5.7 containing 100 mg L-1 timentin, and 350 mg L-1 cefotaxime) for two weeks. The shoot induction medium must be refreshed for an additional period of two weeks (Paz et al., 2004; Song et al., 2013).
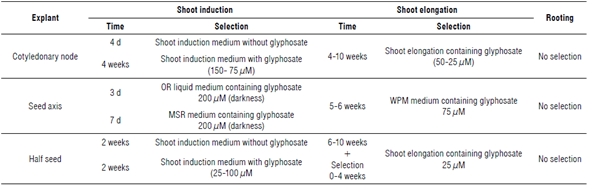
Our experience implies a longer-term evaluation of tolerance by using lower concentrations of glyphosate of 0 mg L-1 for shoot initiation 1 (regeneration 1), then a glyphosate concentration of 25 mg L-1 for shoot initiation 2 (regeneration 2), and then a concentration of glyphosate reduced to 6 mg L-1 (shoot elongation) and 0 mg L-1 (rooting), respectively. Herbicide selection includes shoot induction without glyphosate (0 ^M) (the first two weeks) and 148 (subsequent two weeks). After four weeks in shoot induction medium, explants are transferred into a shoot elongation medium (1X MS salts, 1X Gamborg vitamins, 0.5 mg L-1 GA3, 0.1 mg L-1 IAA, 0.7 mg L-1 BAP, 30 g L-1 sucrose, 3 mM MES, 50 mg L-1 asparagine, 50 mg L-1 glutamine, 70 mg L-1 vancomycin, 350 mg L-1 cefotaxime, 35 glyphosate, 0.7% agar, and pH 5.7). A new culture medium must be prepared every two weeks for six weeks (16/8 h photope-riod, 26°C). Every shoot from the elongation step that has at least 2 cm is moved to the rooting medium (0.66X MS salts, 1X Gamborg vitamins, 3 mM MES, 30 g L-1 sucrose, 0.7% agar, and pH 5.7). The whole process is summarized in Figure 1. Some alternative selection strategies have been described (Clemente et al., 2000; Monsanto Technology, 2011; Dow AgroSciences, 2014) (Tab. 3). We have used a strategy for the transformation and selection of GM events that is consistent with the strategic FTO. Therefore, the reporter genes from pCAMBIA vectors are removed using restriction enzymes and, in this way, the CP4 epsps gene is both a desired trait and gene reporter avoiding unjustified additional IPRs. The use of low concentrations for glypho-sate selection in the regeneration media could increase in vitro escapes (non-germline transformation or chimerism in primary transformants). It is for this reason that a selection marker is important (e.g., antibiotic resistance or green fluorescent protein genes) (Miki & McHugh, 2004). Selection markers have been at the center of controversy about biosafety concerns; therefore, new approaches to produce marker-free GM plants were developed for novel events (Darbani et al., 2007; Tuteja et al., 2012). Since the glyphosate-tolerant GM soybean development has an FTO focus related to the CP4 epsps gene (as mentioned above), the transformation of a high number of explants is essential. The efficiency of soybean transformation can be explained by plant defense mechanisms such as mitogen-activated protein kinases, defense proteins, reactive oxygen species, or hormones (Imam et al., 2016). Soybean transformation has a very narrow efficiency (glyphosate-tolerant events vs. infected explants) of less than ~6% using Agrobacterium (Hinchee et al., 1988), probably since A. tumefaciens has low infective capacity on soybean tissues. Additionally, it is dependent on the soybean genotype and A. tumefaciens strain (Song et al., 2013). These reasons are enough to strongly support the evaluation of a great set of explants, considering the additional loss of material as a result of contamination, seed quality, or genotype resistance.
Transformants that survive long term glyphosate-selection must be transferred to the soil and subjected to leaf painting with glyphosate at 2300 g acid equivalent (ae) h-1 and the tolerance behavior could be scored one week after the treatment. ELISA and herbicide painting analysis must also be conducted in T0 plants (hemizygous dominant) (Passricha et al., 2016), and the phenotypic response must be correlated with molecular analysis. With these new materials, it is possible to obtain a high number of homo-zygous individuals for the trait. Possible GM lines must be evaluated using PCR (transgene presence), ELISA (EPSPS protein), Southern blot (transgene copy number), and real time PCR (transgene expression). The rooted primary transformants must be individualized and propagated in soil for a period of 30 d. The plantlets could be screened with ~0.2% glyphosate (~300 per plant) to assess their survival rate 10 d after aspersion (Guo et al., 2020).
The T-DNA and Vir proteins form a complex that controls the supply of a single T-DNA strand (T-strand) into the plant chromosomes. The assimilation of the T-strand is, probably, due to homology repair, or to non-homologous, or perhaps microhomology-mediated end joining that repairs DNA double-strand breaks. The host DNA structure, tissue expression, and soybean genotypes play a relevant role in transformation efficiency (Hintz et al., 1992; Cober et al., 2009; Jamsheed et al., 2013) (Tab. 2). The presence of 5-25 bp of homology between RB/LB of the T-DNA and the plant genome, the frequent insertion of the T-DNA in promoter regions and gene rich regions, and the correspondence of T-DNA tag density and gene density between GC/AT content provide support for the hypothesis of microhomology-facilitated end joining mechanism, which explains why the T-DNA insertion occurs in several locations (introns, terminators, telomeres, or repetitive sequences) (Jamsheed et al., 2013; Bourras et al., 2015). In the present case, there are several patents that include new approaches to the molecular mechanisms used by A. tumefaciens during plant transformation: WO2007132193A1 (entitled: modified vird2 protein and its use in improved gene transfer), WO2004035731A2 (entitled: increasing host plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis vip1 gene), US20150267213A1 (entitled: strains of Agrobacterium modified to increase plant transformation frequency), WO2016125078A1 (entitled: Agrobacterium-mediated genome modification without T- DNA integration), WO2002052026A2 (describes methods to DNA integration through homologous recombination pathway), and US6800791B1 (an engineered A. tumefaciens strain to transfer proteins to plant cell). This is just a small window on the global patents currently taking place about Agrobacterium mechanisms, and several of these patents are still valid.
Final considerations and conclusion
The adoption of GM herbicide tolerant crops has positive impacts on agriculture, such as an increase in weed control to herbicide management, compared to traditional systems. These GM crops support crop management with a reduction of the impact on the environment and human health due to a lower use of herbicides in the post-emergent phase with beneficial effects on the soil ecosystem. GM crops also favor the adoption of agricultural conservation practices such as the minimum tillage system that reduces soil erosion, improving water quality and soil degradation (Owen, 2010; Green, 2012). Glyphosate is an herbicide widely used; it is of slow action, a fact that facilitates its translocation from leaves to meristematic tissues and makes it environmentally safe. Glyphosate shows slow mobility in the soil, which reduces the likelihood of contamination of local waterbodies, and a relatively short half-life in the soil. Prior to the introduction of GM crops exhibiting tolerance to glyphosate, this herbicide could only be used in areas where no plant growth is desirable, or with methods avoiding contact with commercial crops. This way, GM crops opened up the possibility for direct use by farmers (Duke & Cerdeira, 2010). GM glyphosate-tolerant soybeans simplify weed management. The farmer may control weeds with just a few applications (one or two) of herbicide during the growth cycle instead of using complicated strategies that include different herbicides incorporated into the soil and/or foliar application. The herbicide could only be applied when there is the presence of weeds because of its post-emergent action, promoting the limited use of these kind of products (using less toxic alternatives). Plowing can be reduced or even eliminated, which lowers expenditure on fossil fuels or equipment with a significant reduction in CO2 emissions and soil disturbance (Green, 2012).
A patent protects an invention for 20 years; since 2014, plant patents are to expire shortly, and the appreciation of a new FTO framework is one of the most interesting matters in GM soybean programs towards "generic" events. The implementation of an FTO analysis must consider the Cartagena Protocol on Biological Diversity that was adopted on September 11, 2003, and the Nagoya-Kuala Lumpur Supplementary Protocol on Liability and Redress to the Cartagena Protocol on Biosafety that was effective on October 15, 2010 (Keiper & Atanassova, 2020). These protocols established that a GM variety should demonstrate biological efficacy, agronomic efficiency, no side-effects on non-target populations, absence of gene flow, and safety for the consumers (Castaño Hernández, 2013; Chaparro-Giraldo, 2013). When it comes to advancing technology with a long history of safe use in different national jurisdictions, there is still a large legacy of scientific articles or official documents that are important for business (McHughen, 2012). This is the case of the Monsanto/Bayer event GTS 40-3-2 that has been released in 27 countries since 1994. The agro-generics strategy is a model than could be used for bolstering administrative efficiency and reducing costs. According to McDougall (2011), based on company data (BASF, Bayer, Dow, Dupont, Monsanto/ Bayer, and Syngenta), approximately 13 years and USD 136 million are spent to develop a GM variety: USD 31 million (23%) on gene discovery, USD 69.9 million (51%) on product development, and USD 35.1 million (26%) on regulation and registration. However, Schiek et al. (2016) report expenditures of USD 1.6 million and eight years for the development of a potato GM variety by public institutions in underdeveloped countries.
This article offers an approach that is not only limited to the experimental area but also provides a view for an FTO application based on how the development of a GM crop might be understood and planned to increase the likelihood of achieving the desired market result. However, in some cases, it is also possible that legal support might be needed to negotiate some licenses. The soybean genotype, cloning vectors, genetic construct, A. tumefaciens strain, selection strategy, reporter gene usage, or in vitro protocols were identified as key elements for attaining the objectives defined for the GM development concerned. At the end of this process, it was possible to notice that Google patents has become an important tool for enhancing patent revision for this topic.
In summary, the GM soybean for glyphosate tolerance experimentation needs to be carefully planned and prioritized, and all procedures and conditions must be optimized to ensure quality efficiency. Patents that could prevent access to this technology need to be identified and negotiations with relevant parties regarding a GM soybean for glyphosate tolerance project should be carried out on a case-by-case basis depending on the experimental phases. Finally, it is necessary to consider legislation on regulatory aspects of biosecurity.