Introduction
The field pea (Pisum sativum L.) belongs to the Fabaceae family and is one of the oldest domesticated species for human and livestock consumption. It is widely cultivated around the world (Wu et al., 2019) and predominant in world trade, representing about 35-40% of the total trade in legumes. It is also among the most consumed vegetables worldwide (Ratnayake et al., 2001). The pea is an important nutritional crop (Liu et al., 2015) since its grains have high contents of protein (18-30%), vitamins and minerals. Additionally, its shoots and leaves can be used for fresh consumption as leafy vegetables (Santos et al., 2014), with a good demand in the national and international markets. Field pea is a profitable agricultural product. However, this profitability is diminished in many regions by various factors such as environmental problems, incorrect agronomic management, and increase in production costs due to the rise in the application of fertilizers and pesticides (Erisman, 2011). Agronomic management is especially critical because long-duration varieties with low yields are still sown in production areas and are susceptible to different diseases and pests (Wu et al., 2019). For this crop, correct physiological and nutritional management is imperative, with biostimulants and plant hormones playing an important role (Bertolin et al., 2010; Cato et al., 2013; Martínez González et al., 2017).
Plant growth regulators are small molecules that can trigger different physiological processes related to the growth, development, and defense of plants (Pozo et al., 2015). They are also known as phytohormones, but this term is not used frequently in agriculture (Davies, 2010). Plant hormones, growth regulators, and inhibitors have been used in practice to increase yield, improve quality, or alleviate the adverse effects induced by biotic or abiotic stresses (Csukasi et al., 2009). Different classes of hormones have already been characterized, including abscisic acid, auxins, brassinosteroids, cytokinins, ethylene, gibberellins, jasmo-nates, strigolactones, etc. (Depuydt & Hardtke, 2011). All of them have been linked in one way or another to growth regulation (Santner et al., 2009; Wolters & Jürgens, 2009). Cytokinins, auxins, gibberellins, and brassinosteroids are considered essential for growth of mutant phenotypes in which hormone biosynthesis or perception is disrupted; cytokinins regulate cell proliferation, while gibberellins promote cell elongation and auxins are involved in both processes. Furthermore, brassinosteroids are essential for cell elongation, but may also play a role in cell division (Nakaya et al., 2002; Hardtke et al., 2007). All these hormones can regulate a high number of processes in a unique and independent way. However, cooperation and interrelationship between signaling metabolic pathways appears to exist, as it follows from the superimposed influence on various cellular processes (Hardtke et al., 2007).
New hormones and plant growth regulators are still being discovered, and their interrelationships, mechanisms of action, and relationships at the metabolic level are being exhaustively studied (Gomez-Roldan et al., 2008; Kuppusamy et al., 2009). Among these substances is triacontanol (TRIA), a growth regulator recently used in commercial applications despite the fact that it was discovered a few decades ago in natural waxes. It can exert stimulating effects, even at considerably low foliar concentrations (Khandaker et al., 2013). TRIA is not considered a plant hormone since it is a secondary substance in plant growth (Naeem et al., 2012). However, various studies show that its effects on growth and yield in plants are not shown by other plant hormones or growth regulators. The positive role of TRIA in photosynthesis, nitrogen fixation, enzymatic activities, free amino acids, reducing sugars, and soluble proteins in plants has been documented (Borowski et al., 2000; Naeem et al., 2009; 2010; 2011; Aftab et al., 2010; Khandaker et al., 2013). The application of TRIA also increases the dry weight, chlorophyll content, protein and net photosynthetic efficiency in rice (Oryza sativa L.) (Chen et al., 2002). In cotton, it promotes vegetative growth and increases the level of monogalactosyldiacylglycerol (MGDG), a galactolipid that appears to be involved in the synthesis of photosystem I proteins (Naeem et al., 2012). The application of TRIA alone or in combination with potassium increases plant height, fresh and dry weight of the plant and leaf area in tomato (Khan et al., 2009).
In this exploratory study, we evaluated the effect of the interaction of plant growth regulators with triacontanol on the field pea Pisum sativum L. cv. Rondo to determine the roles of growth regulators applied alone and in combination and their impact on yield components and plant growth and development.
Materials and methods
The experiment was carried out in the experimental fields of the Instituto Nacional de Innovación Agraria (INIA), La Molina, Lima, Peru (12°04'48" S, 76°56'44" W at 243 m a.s.l.) between September and December 2015. To determine the effect of the application of triacontanol (TRIA) and the growth regulators on peas, pea seeds cv. Rondo were used. Rondo is a commercial cultivar with a determinate growth habit, semi-late, with straight pod and rough grain suitable for fresh consumption, and with medium maturation appreciated by farmers for its yield and pod size. Climate characteristics of the field site are described in Table 1. Four pea seeds were sown per hole under drip irrigation at a distance between plants of 0.75 x 0.2 m, with 24 experimental plots of 9 m2 and a total area of 320 m2 (experimental plots + edge spaces). Average soil characteristics of the field site were: sand, 53%; silt, 28%; clay, 19%; organic matter, 1.12%; P, 17.4 mg kg-1; K, 291 mg kg-1; pH, 7.7, and electrical conductivity, 1.62 dS m-1. The soil analysis was carried out according to the guidelines established by Burt (2014). Subsequently, manual thinning was done, leaving three plants per stroke and a final planting density of 200,000 plants ha-1.
TABLE 1 Average climate data during the crop cycle.
| Month | Maximum temperature (°C) | Minimum temperature (°C) | Relative humidity (%) | Mean precipitation (mm/month) |
|---|---|---|---|---|
| September | 21.52 | 15.34 | 80.21 | 0.12 |
| October | 22.77 | 16.16 | 79.75 | 0.06 |
| November | 22.9 | 16.64 | 79.33 | 0.09 |
| December | 25.08 | 17.97 | 77.92 | 0.06 |
To evaluate the effect of the treatments on growth and yield, the plant height, number of branches per plant, plant fresh weight, pod length, pod width, number of pods per plant, number of grains per pod and green pod yield (t ha-1) were determined at harvest.
At 43 d after sowing (V4 growth stage), the treatments described in Table 2 were foliar applied. All treatments, except the control, alone or in combination with other plant growth regulators (auxins (AUX), gibberellins (GA) or cytokinins (CK)) contained TRIA, and a foliar-applied potassium fertilizer (K). Other commercial foliar fertilizers from FARMEX S.A. (Peru), such as FX Amino and Powergizer, were also applied to all treatments at the same concentration to replace nitrogen deficiencies.
TABLE 2 Description of the treatments and doses of the plant growth regulators used in the experiment.
| Name | Description |
|---|---|
| TRIA+AUX | 0.5 mg L-1 triacontanol + 10 mg L-1 NAA |
| TRIA+GA | 0.5 mg L-1 triacontanol + 10 mg L-1 GA3 |
| TRIA+CK | 0.5 mg L-1 triacontanol + 1 mg L-1 KIN |
| TRIA+AUX+CK | 0.5 mg L-1 triacontanol + 0.5 mg L-1 NAA + 1 mg L-1 KIN |
| TRIA+AUX+GA+CK | 0.5 mg L-1 triacontanol + 0.5 mg L-1 NAA TRIA+AUX+CK + 1 mg L-1 KIN |
| TRIA+K | 0.5 mg L-1 triacontanol + 3000 mg L-1 K2O |
| TRIA | 0.5 mg L-1 triacontanol |
| CONTROL | Treatment without any application of triacontanol or growth regulators |
NAA - 1-Naphthaleneacetic acid; GA3 - gibberellic acid; KIN - kinetin; K2O - potassium oxide.
A completely randomized block design was used, with eight treatments and three replicates, for a total of 24 experimental units. The results were subjected to a normality test and analysis of variance (ANOVA) by the F test, and the means were compared with the least significant Fisher's test (LSD) at 5% probability of error, using the statistical software R. Pearson's correlation test at P≤0.05 was applied to assess the significance of correlation coefficients.
Results and discussion
Growth parameters
The plant growth regulators applied in this study caused changes in plant height. The application of TRIA + GA obtained the highest plant height (143.5 cm), and the control obtained the lowest value (79.8 cm), which was 15.6% lower than the average obtained in the experiment (Tab. 3). The height of the pea plant is a characteristic determined by its genetics (Weeden, 2007), and the interaction with growth-promoting phytohormones such as GA (Wang et al., 2017). The control of this experiment showed a height greater than that reported by Anchivilca Rojas (2018) (68 cm), Santos et al. (2018) (60 cm), and Rodríguez Quispe (2015) (52 cm). According to Checa Coral et al. (2020), this could be due to environmental conditions and an efficient supply of resources since the resulting phenotype is the interaction of the genotype and the environment. The positive interaction of TRIA and GA agrees with that proposed by Shukla et al. (1992), who mention that triacontanol potentiates the effect of GA. The opposite is observed with AUX, since TRIA decreases its influence as a "destruction" effect is produced. The intensity of this effect is specific in each cultivar (Henry & Gordon, 1980); auxins at low concentrations inhibit stem growth in favor of root growth and development (Davies, 2010; Taiz et al., 2014). No effects were observed with foliar application of potassium because the soil was rich in this element (291 mg L-1) and the application of potassium would keep the plants under conditions of "luxury consumption" (Marschner, 2011).
TABLE 3 Effect of plant growth regulators on growth and yield of pea plants.
| Treatment | PH (cm) | NBP | PL (cm) | PW (cm) | NPP | NGP | PFW (g) | Yield (t ha-1) |
|---|---|---|---|---|---|---|---|---|
| TRIA+AUX | 86.5b ± 8.3 | 2.7ab ± 0.4 | 9.67ab ± 0.7 | 1.77ab ± 0.03 | 9.5abc ± 1.0 | 6.1c ± 0.7 | 130.2bc ± 21.5 | 7.43c ± 0.8 |
| TRIA+GA | 143.5a ± 4.6 | 2.2b ± 0.3 | 8.26b ± 0.4 | 1.83ab ± 0.09 | 3.7c ± 0.9 | 4.6d ± 0.01 | 93.5c ± 11.5 | 2.15d ± 0.3 |
| TRIA+CK | 95.8b ± 6.4 | 2.8ab ± 0.2 | 10.67a ± 0.1 | 1.74ab ± 0.03 | 12.2ab ± 3.4 | 7.9ab ± 0.3 | 143.3abc ± 15.6 | 8.47bc ± 0.9 |
| TRIA+AUX+CK | 85.0b ± 6.1 | 2.8ab ± 0.2 | 10.29a ± 0.4 | 1.68ab ± 0.02 | 15.0a ± 1.5 | 7.7ab ± 0.2 | 154.5ab ± 7.2 | 10.53ab ± 0.9 |
| TRIA+ AUX+GA+CK | 91.5b ± 4.5 | 3.5a ± 0.5 | 10.71a ± 0.7 | 1.83a ± 0.06 | 14.7ab ± 3.2 | 8.7a ± 0.2 | 198a ± 34.9 | 12.15a ± 1.4 |
| TRIA+K | 85.7b ± 3.3 | 2.7ab ± 0.2 | 10.46a ± 0.4 | 1.81ab ± 0.03 | 9.0bc ± 1.3 | 7.7ab ± 0.6 | 120.8bc ± 15.4 | 7.30c ± 1.0 |
| TRIA | 88.7b ± 3.3 | 2.7ab ± 0.2 | 10.21a ± 0.3 | 1.73ab ± 0.05 | 12.8ab ± 0.9 | 7.2bc ± 0.4 | 135.8bc ± 9.3 | 8.88bc ± 0.4 |
| CONTROL | 79.8b ± 7.2 | 2.8ab ± 0.2 | 9.33ab ± 0.5 | 1.66b ± 0.04 | 13.3ab ± 1.5 | 5.9cd ± 0.3 | 122.2bc ± 14.6 | 7.98bc ± 1.0 |
| F-Test | ** | ns | ns | ns | * | ** | ns | ** |
| Mean | 94.56 | 2.77 | 9.95 | 1.75 | 11.27 | 6.98 | 137.29 | 8.11 |
TRIA - triacontanol, AUX - auxins, GA - gibberellins, CK - cytokinins, K - foliar-applied potassium, PH - plant height, NBP - number of branches per plant, PL - pod length, PW - pod width, NPP - number of pods per plant, NGP - number of grains per pod, PFW - plant fresh weight. ns - not significant at P>0.05; *: P≤0.05; **: P≤0.01.
Regarding the number of branches per plant, there were no statistical differences in the analysis of variance for the treatments. Most of the treatments obtained similar values to those of the control, except for TRIA+GA, with a value 21.8% less than the average, and TRIA+AUX+GA+CK, with a value 23.5% greater than the control and 26.3% greater than the average (Tab. 3). The trend is repeated for pod length and plant fresh weight regarding the highest and lowest values in the treatments. However, for pod width, the control showed the lowest value of 1.66 cm, TRIA+AUX showed an intermediate value of 1.76 cm, and TRIA+AUX+GA+CK obtained the highest value with 1.83 cm.
The number of branches plant (NBP) and the pod width are also genetically determined characteristics, but the former is more susceptible to variations due to environmental conditions and/or agronomic practices than the latter (presence of the n gene, Wehner & Gritton, (1981)). Pod width did not exhibit significant or percentage statistical variation from all variables, showing a less marked incremental effect of the negative interaction of TRIA with AUX and CK applied jointly (Fig. 1). On the other hand, for NBP, the treatments with CK increased the branching of the shoots (Müller & Leyser, 2011) and the number of branches in the plants (Taiz et al., 2014).
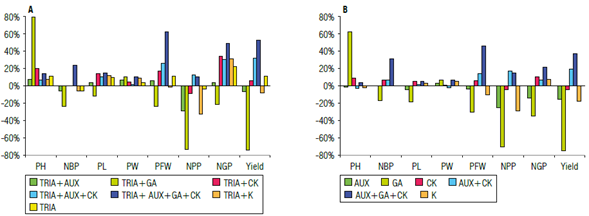
FIGURE 1 Differential effects of the application of A) triacontanol (TRIA) compared to the control and B) the plant growth regulators (AUX - Auxins, GA - Gibberellins, and CK - Cytokinins) alone or mixed compared to triacontanol. PH - plant height; NBP - number of branches per plant; PL - pod length; PW - pod width; NPP - number of pods per plant; NGP - number of grains per pod; PFW - plant fresh weight.
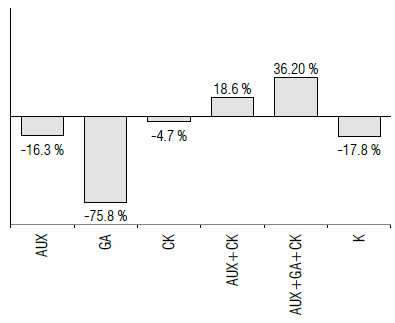
Pearson's correlation coefficients showed interesting data regarding the relationships between variables. Within the vegetative parameters, plant height was negatively correlated with pod length (-0.271) and the number of branches per plant was positively correlated with pod length (0.442*) and yield (0.731**). The variable pod length was positively correlated with plant fresh weight (0.626**), number of grains per pod (0.823**), and green pod yield (0.654**). A positive correlation was also observed between these last three variables. However, these variables were not positively correlated with pod width (Tab. 4). In contrast with the values obtained in the variable plant height, the average pod length in this experiment (9.9 cm) was lower than that obtained by Anchivilca Rojas (2018) (10 cm), Rondinel Ruíz (2014) (10.2 cm), and Rodríguez Quispe (2015) (11.4 cm). Triacontanol application resulted in an increase of 11.3% in yield over the control (Fig. 2). A positive interaction was also observed with treatments containing CK and, to a lesser extent, with foliar potassium. A slight negative interaction was observed with AUX and a higher negative interaction was registered with gibberellins.
TABLE 4 Pearson's correlation coefficients of the variables tested.
| PH | NBP | NPP | PL | PW | PFW | NGP | |
|---|---|---|---|---|---|---|---|
| NBP | -0.271 | ---- | ---- | ---- | ---- | ---- | ---- |
| NPP | -0.573** | 0.655** | ---- | ---- | ---- | ---- | ---- |
| PL | -0.443* | 0.442* | 0.396 | ---- | ---- | ---- | ---- |
| PW | 0.225 | 0.243 | -0.232 | 0.131 | ---- | ---- | ---- |
| PFW | -0.255 | 0.891** | 0.670** | 0.626** | 0.267 | ---- | ---- |
| NGP | -0.447* | 0.456* | 0.485* | 0.823** | 0.058 | 0.587** | ---- |
| Yield | -0.613** | 0.731** | 0.883** | 0.654** | -0.009 | 0.845** | 0.723** |
PH - plant height; NBP - number of branches per plant; NPP - number of pods per plant; PL - pod length; PW - pod width; PFW - plant fresh weight, and NGP - number of grains per pod.
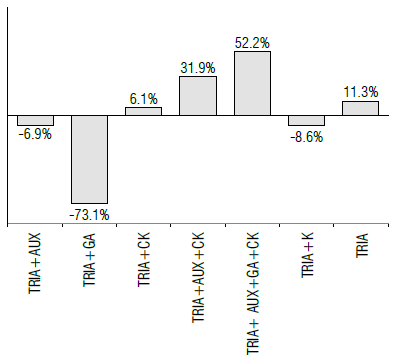
FIGURE 2 Differential effect of the treatments on the yield (t ha1) compared to the control. TRIA - triacontanol, AUX - auxins, GA - gibberellins, CK - cytokinins, K - foliar-applied potassium.
The effect of CK is significant since they promote greater pod setting in legumes (Carlson et al., 1987) and increase the width, length, and weight of pods (Mosjidis et al., 1993). Potassium also has a slight incremental effect on fruiting and a higher pod setting in legumes (Marschner, 2011). However, this is not reflected by a luxury consumption of K. The trend of the effect of the hormonal interaction is repeated on pod length, as well as on plant fresh weight, except that a negative interaction with foliar potassium was also recorded. Although gibberellins favor the vegetative growth of the plant when applied in excess (Taiz et al., 2014), this growth is very fast, forming tall but weak stems with lower dry matter content and fresh weight of the plant. The mixture of the three hormones considerably increases the plant fresh weight due to a better regulation of physiological processes and the internal hormonal balance in the plants (Figs. 1-3).
Yield parameters
The lowest values of all yield variables (number of pods per plant, number of grains per pod, and green pod yield) were obtained with TRIA+GA. The highest values were recorded with TRIA+AUX+GA+CK, in which all the hormones were used, except in the number of pods per plant, where the best treatment was TRIA+AUX+CK (Tab. 3).
The number of pods per plant showed a different behavior between treatments, although it was highly positively correlated with the number of grains per pod (0.485*) and yield (0.883**) as shown in Table 3. An increase of 308.7% over the lowest value of this variable and 12.52% over the control was recorded. Statistically significant differences between the treatments were observed, since a very strong detrimental effect of GA was seen in this variable. Figures 1A and 1B show the positive interaction of TRIA with the mixture of AUX, CK and GA, but a negative interaction with AUX and CK applied separately.
Regarding the number of grains per pod, significant statistical differences were observed between the treatments.
The TRIA+AUX+GA+CK showed an increase of 48.8% over the control and 14.3% over the TRIA applied alone, which, in turn, represents a 22.6% increase over the control.
TRIA+AUX, TRIA+GA and the control did not exceed the average value obtained in the experiment of seven grains per pod (Tab. 3).
Within the yield components, the number of pods per plant with the application of TRIA decreased by 3.8% compared to the control (Fig. 1A). No positive interactions were observed with the mixture of AUX and CK or with the mixture of the three hormones. However, a negative interaction was recorded with AUX and CK applied separately since the AUX-CK relationship is imbalanced in physiological processes. A large decrease was observed with GA since they favor vegetative growth (Davies, 2010), and foliar potassium application obtained values below the control (32.5% less).
The number of pods per plant is one of the most important yield components in legumes. GA decreased this yield variable because it promotes growth to the detriment of fruiting and formation of plant flowers (Davies, 2010). CK promote pod formation and show synergism with auxins (Cato et al, 2013). The positive impact of CK has been observed in plants, such as Artemisia grown in vitro (Rasool et al., 2013), showing a greater effect with the mixture of the three growth regulators (Cato et al., 2013).
Foliar-applied potassium has a positive effect on the control and an interaction with TRIA, similar to that occurring with CK. Regarding the variable green pod yield, the values obtained ranged between 2.15 and 12.151 ha-1 with highly significant statistical differences between treatments (P<0.01). TRIA+AUX+CK and TRIA+AUX+GA+CK exceeded 10 t ha-1, and TRIA+GA showed a decrease of 73.1°% relative to the control. TRIA+AUX and TRIA+K did not exceed the control. The application of TRIA alone showed an increase of 11.3% relative to the control (Fig. 2).
Pearson's correlation coefficients of the yield variables showed that the number of pods per plant was positively correlated with number of grains per pod (0.485*), number of branches per plant (0.655**), plant fresh weight (0.670**), and yield (0.883**), and negatively correlated with plant height (-0.573**). The number of grains per pod was also positively correlated with the other variables (P<0.05). The same was observed for the variable green pod yield, except for pod width, which is not correlated with any variable (Tab. 4).
Green pod yield is the agronomic trait that helps to determine the profitability of the crop. It also determines if the crop is a good alternative for the farmer, who looks for high yields with increasing emphasis on quality (Espinosa & Ligarreto, 2005; Checa Coral et al., 2017). The high yield values obtained are due to the fact that the values of number of branches per plant, number of pods per plant, and number of grains per pod were also higher. These last two variables are important components of the yield and determine the productivity of plants. The lowest values were obtained in the interaction of TRIA with AUX and GA applied separately. This shows the negative effect of GA on crop yield in general since they decrease pod formation favoring vegetative growth and increasing plant height, an unfavorable issue leading to lodging (Davies, 2010; Hedden, 2016; Wang et al., 2017). It also shows the negative effect of AUX applied alone, since they alter the AUX-CK relationship that is key to the growth and development of plant species (Schaller et al., 2015).
TRIA is a natural growth regulator widely studied in various species, with notable effects on the growth and development of rice (Jadhav et al., 2017), Capsicum annuum L. (Sahu et al., 2017), willow (Digruber et al., 2018), sweet potato (Rajak et al., 2018), strawberry (Baba et al., 2017), and guava (Singh et al., 2017). Pearson's correlation coefficients show a highly positive correlation between yield and the number of pods per plant (0.883**), number of branches per plant (0.731**), number of grains per plant (0.723**), plant fresh weight (0.845**), and pod length (0.654**) (Tab. 4).
The differential effect for TRIA in this experiment was greater, especially in the yield components, with an increase in plant height of 11%, a decrease in number of branches per plant and number of pods per plant of 5.9 and 3.8%, respectively, and increases of 9.5% in pod length, 4.2% in pod width, 11.19% in plant fresh weight and 22.6% in number of grains per pod.
Conclusions
The results of this study showed that TRIA by itself has no remarkable effect on the growth and yield of Pisum sativum; only when TRIA is combined with other plant growth regulators such as AUX, GA and CK are noteworthy effects seen. AUX, GA and CK increased morphological variables with the exception of the number of branches per plant, and pod length in the case of GA. Within the yield components, AUX and CK increased the number of grains per pod and GA decreased all these variables, showing a negative interaction with TRIA. AUX and CK acted synergistically in almost all the variables evaluated, except for the number of branches for which similar values were obtained. In all these variables, the synergistic action of AUX and CK exceeds the effect of each growth regulator separately. The joint application of foliar-applied potassium fertilizer with TRIA showed an unfavorable effect on the yield and plant fresh weight. The highest green pod yields were obtained with the application of TRIA plus AUX, GA and CK (12.15 t ha-1), followed by the application of TRIA plus AUX and CK (10.53 t ha-1). The trihormonal application with TRIA obtained the best results in the variables because the hormonal relationship is maintained and is not altered by the imbalance of the others.