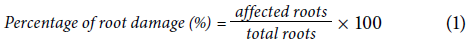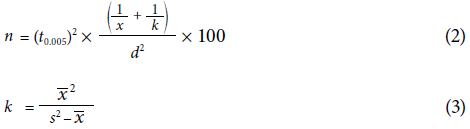Introduction
The department of Santander has the highest pineapple production in Colombia, with 11,444 ha planted and a yield per area below the national average (Ministerio de Agricultura y Desarrollo Rural, 2017). Two pineapple materials predominate, the traditional variety Perolera and the hybrid MD2, also called "Oro miel". The latter has increased in planted area in recent years and has a higher market price. However, the variety Perolera is widely accepted by farmers, has a market for agribusiness, and a higher tolerance to pests and diseases, according to growers. Although, there is information about arthropods associated with the variety Perolera (Morales Granados & López González, 2002), little is known about the susceptibility of the hybrid MD2 to pest arthropods due to its relatively short introduction.
During the pineapple vegetative growth phase and especially the first five months after planting, symphylids (Class Symphyla) are of special importance (Rohrbach & Johnson, 2003). They are thin, whitish arthropods between 1 - 8 mm long, and the adults have 12 pairs of legs and long antennae (Domínguez Camacho, 2015). They are generally associated with detritivore and omnivorous habits and there are few records of agricultural importance (Gerdeman & Diehl, 2021). In pineapple plants, symphylids consume tender roots and induce the formation of very short and not very functional roots that impede adequate nutrition, delay growth, and affect the anchoring of the plant (Saavedra, 1990; León, 1997). Due to damage by symphylids, a reduction of up to 67% of the roots fresh weight is reported under pot conditions (Agredo et al., 1988) and up to 70% in pineapple crops (Morales Granados & López González, 2002). They can also favor the proliferation of root diseases (Saavedra, 1990; Castañeda, 1998). Despite their importance, many aspects of the biology and ecology of neotropical symphylids are unknown, and this information could be the key to improving management and control practices in favor of less polluting and more sustainable procedures.
Symphylids have an aggregate distribution (Soler et al., 2011). Therefore, locating their colonies within the crop would be the first step for their management. This activity is difficult because the symptoms caused by their damage, such as growth retardation and the reddish coloration of leaves, can also be confused with the damage of mealybugs (Agredo et al., 1988; García Reyes, 1994). Symphylid monitoring has been mainly based on uprooting pineapple plants and observing their presence in the soil and roots (Morales Granados & López González, 2002). This destructive method can be inaccurate and difficult to standardize, making it hard to use as a decision tool. A new sampling methodology based on traps with a bait of potato pieces and soil has shown to be efficient and easy to use (Soler et al., 2011). However, its benefits have been neither evaluated nor compared with the traditional sampling methodology, nor has this method been related to the damage of symphylids in pineapple roots.
The objectives for this study were i) sampling and monitoring symphylids associated with pineapple plantations on the vegetative growth stage, ii) comparing the efficiency of two sampling methods and their relationship to root damage caused by symphylids, and iii) evaluating the influence of soil pH and moisture on the presence of symphylids.
Materials and methods
Selection of commercial pineapple plots
Five farms were selected in the pineapple growing area of the municipality of Lebrija, Santander with plots close to being planted, recently planted, or two weeks after planting, with the aim of monitoring symphylids in the vegetative growth phase (Tab. 1). Depending on the availability, they were searched so that the traps in both varieties were well distributed in the region.
TABLE 1 Sampled pineapple farms of the municipality of Lebrija, Santander.
| Rural district - farm | Georeferenced position | Altitude (m a.s.l.) | Variety/hybrid (n) | Planted area |
|---|---|---|---|---|
| La Aguada - El Remolino (REM) | 7°11'42.1"N 73°10'22.8"W | 834 | Perolera (10) | 2 ha |
| La Aguada - La Esperanza (ESP) | 7°12'03.8"N 73°10'24.2"W | 905 | MD2 (10) | 2 ha |
| La Aguada - Hoya Larga (HLA) | 7°11'52.9"N 73°10'03.6"W | 970 | Perolera (5) and MD2 (5) | 3 ha |
| La Aguada - El Diviso (DIV) | 7°11'48.16"N 73°10'17.2"W | 973 | MD2 (10) | 6 ha |
| La Puente - La Trinidad (TRI) | 7°04'44.3"N 73°12'12"W | 1317 | Perolera (15) and MD2 (5) | 4 ha |
The values of (n) indicate the number of sampling points.
Identifying and monitoring symphylids with bait traps
Bimonthly monitoring was carried out from the planting of the crop or a few weeks later to 8 months after planting, for a total of five samplings. In each farm, 10 to 20 symphylid traps were set within the pineapple crop with a separation between traps of at least 20 m. According to Soler et al. (2011), symphylid colonies have a width of between 4 - 6 m, and they are stable over time with little lateral displacement (Gerdeman & Diehl, 2021). Under these circumstances, a distance of 20 m was established to ensure that the traps were independent of each other. At each sampling time, 60 traps were installed, 30 for the variety Perolera and 30 for the hybrid MD2. The traps were installed at the same sampling points at each sampling time.
The symphylid traps consisted of plastic containers with perforations (Fig. 1A), to which potato pieces mixed with soil were introduced as bait; they were then buried and checked after 3 d (Fig. 1B). The traps had the same capacity (250 ml) as those originally proposed by Soler et al. (2011) and with enough perforations to expose the bait to the symphylids.

FIGURE 1 Symphylid sampling methodology. A) PVC plastic container used for sampling. B) Container with 50 grams of potato used as bait. C) Collection of plants. D) Bifurcation in the roots caused by symphylid damage.
The traps were transferred to the laboratory of La Suiza Research Center of the Colombian Agricultural Research Corporation - AGROSAVIA. There, they were thoroughly checked, spreading the soil and the bait in a dark-colored plastic tray where the captured specimens were collected with brushes and deposited in 75% ethyl alcohol.
All symphylids collected were placed into labeled alcohol vials. They were identified to genus with the taxonomic key of Scheller and Adis (1996) for the neotropical region. To compare the number of symphylids between sampling times for each farm, the non-parametric Kruskall-Wallis test and Mann-Withney pairwise comparison, Bonferroni corrected, were used in the PAST program version 1.86b (Hammer et al., 2001). The collection of arthropods was carried out under the collecting permit 1466 of 2014 granted by the National Authority of Environmental Licenses (ANLA).
Comparison of sampling methods and their relationship with symphylid damage
The sampling with the destructive method consisted of taking the closest pineapple plant to each bait trap for each sampling time, trying to collect the root with approximately 1 kg of the surrounding soil (Fig. 1C). These roots and the soil were thoroughly inspected. Symphylids were identified as previously explained for the bait trap method. The soil was weighed to obtain the number of symphylids kg-1 of soil.
The damage caused by symphylids was quantified for each plant and measured as a percentage of affected roots. The affected root was estimated by the apex damage and subsequent bifurcation (Fig. ID). This damage has been reported to be exclusively caused by symphylids (Agredo et al., 1988; Soler et al., 2011). Although it would have been desirable to evaluate a higher number of roots for this study, the number of roots per plant was low and only 10 were measured. The percentage of damage per sampling point was estimated using Equation 1:
To evaluate which of the two symphylid sampling methods was more efficient to estimate population density with fewer samples, the following equations proposed by Soler et al. (2011) were used:
where n represents the necessary number of samples to estimate the symphylid density population in each farm and at each sampling time; t is the aggregation coefficient for a population that has a binomial distribution; t 0.05 is the t-student distribution value, and d the acceptable deviation from the population mean which in this case is 0.25, that is 25%. This analysis was performed only on farms with 10 or more samples of the same variety/hybrid.
Spearman's rank correlations coefficient (ρ) matrix was also carried out between the percentage of damage and the current and previous number of symphylids collected with the two sampling methods four months after planting. This was not done before because the plants had not rooted. This analysis sought to evaluate which of the two methods provided a better predictor of the percentage of root damage and to evaluate if the damage found was old or caused several weeks before. In that case, it would be more related to the symphylid registries of the two previous months than with the recent ones. The time after planting and plant weight were also added to the analysis. Additionally, symphylids in traps and symphylids captured by the destructive method were also correlated for each sampling time: 4, 6, and 8 months after planting, using the PAST program version 1.86b (Hammer et al., 2001).
Relationship between soil pH and moisture and symphylid abundance
In three of the sampling times, one at planting, four months after planting, and at the end of the trial eight months after planting, the pH was measured using a potentiometer, and soil moisture was estimated by heating a sample of 20 g of soil taken from the same trap hole, 30 cm deep, in a microwave oven for 12 min, according to the methodology of Kramarenko et al. (2016).
To evaluate the relationship between pH and the percentage of soil moisture and symphylids, a correlation analysis was performed as previously described.
Results and discussion
Identification and monitoring of the incidence of symphylids
In general terms, symphylid abundance increased from planting to the end of the establishment of the crop eight months later (Fig. 2). The differences between farms in terms of the variations in the populations of symphylids were notable (Fig. 2). The context: slope level, resources, and farmer experience of each farm determined differences in the management practices that most affected the symphylids, such as plowing and pest management. A better understanding of the variations in their populations could be achieved if each farm were analyzed independently.
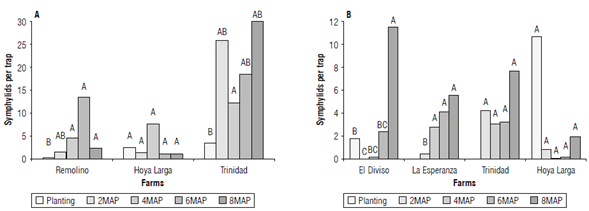
FIGURE 2 Symphylids in the establishment stage of the pineapple crop collected with traps with potato bait + soil. A) Variety Perolera; B) hybrid MD2. MAP - months after planting. Same letters indicate not statistically significant differences according to the Kruskall Wallis test and Mann-Withney pairwise comparison, Bonferroni corrected. For the hybrid MD2, there was no information on the captures of symphylids at the time of planting in the farms La Esperanza and Trinidad.
In all farms, land preparation for planting included plowing and burning, two practices that decrease the populations of symphylids (Gerdeman & Diehl, 2021). After that, populations of symphylids can increase up to five times in only two months (Fig. 2). Symphylids migrate vertically and, although they are superficially concentrated in the first 15 cm, they can go deeper up to 90 cm depending on soil conditions such as structure and water storage capacity, thus avoiding the effect of burning and plowing (Umble et al., 2006; Gerdeman & Diehl, 2021). This way, surviving symphylids can recolonize the soil surface, coming from below after soil preparation (Sarah, 1990). Despite the benefits of plowing as a method of symphylid control, its practice is debatable because pineapple crops are located on land with slopes between 10% and 60% (García Reyes, 1994), where soil conservation practices such as minimal tillage would be recommended to decrease erosion.
Also, the use of pesticides at planting is a widely used and effective strategy for managing symphylids (Agredo et al., 1988; Gerdeman & Diehl, 2021). Soler et al. (2011) record the absence of symphylids for up to four months after edaphic applications of insecticides with the active ingredient ethoprophos at the time of planting. Although ethoprophos has also been recommended in the growing area of Santander (Morales Granados & López González, 2002), farmers prefer to apply insecticides, mainly chlorpyrifos, in drench application at the time of planting or a few weeks later. However, other active ingredients are also used such as carbofuran, acephate, and thiamethoxan. These applications are more efficient at controlling the pineapple mealybug Dysmicoccus brevipes than symphylids, matching the farmer perception that symphylids are not a major pest in their crops. As a result, symphylid populations are maintained or increased a few weeks after applications, demonstrating the low efficacy of these products in controlling symphylids (Fig. 2).
The results of the taxonomic identification confirmed that the collected symphylids belong to the genus Hanseniella. Although Scutigerella immaculata (Newport) has been repeatedly recorded in the growing region of Santander (Morales Granados & López González, 2002) and other regions such as the eastern plains and Valle del Cauca (Agredo et al., 1988; León, 1997), this species was not found in the samplings.
Comparison of sampling methods and their relationship to symphylid damage
Fewer specimens were collected with the destructive symphylid sampling method, while symphylids were captured in 70% of the samples with the traps. Using destructive sampling, they were only found between 12% and 19%. Checking the soil around plants is a destructive and inaccurate method since symphylids are quick and removing the soil alerts them to easily escape from the sample. Other factors such as daytime and the observer's skill can be crucial in generating bias. All these factors decrease the sensitivity of the destructive method and underestimate symphylid populations.
Both sampling methods performed a sample variance higher than their average, except in cases where the records are null or very few, showing that the distribution of symphylids is aggregated (Soler et al., 2011). In most cases, sampling with bait traps requires fewer samples than the destructive method (Tab. 2). In the two methods, a similar time was spent per sampling point, so it was expected that sampling with traps would have values closer to the population mean when using the two methods (destructive and traps) with the same sample size or sampling effort.
TABLE 2 Values of means, variance, dispersion coefficients (k), and number of samples necessary to sample symphylids (n) at four farms in the municipality of Lebrija, Santander.
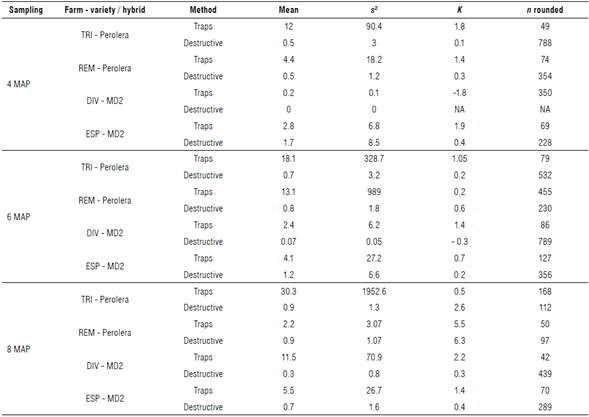
MAP - months after planting. Farm names: TRI - La Trinidad, REM - El Remolino, DIV - El Diviso, and ESP - La Esperanza.
The correlation matrix shows that root damage is related to sampling with bait traps and not to the destructive method (Tab. 3). The previous reading of symphylids with traps two months before was decisive in the percentage of damage. This may indicate that part of the damage registered was old damage that occurred several weeks before. Roots that are no longer functional because of symphylid attacks may take several weeks to decompose. Therefore, the registry of symphylids that is carried out at a certain moment will serve more to predict future damage to the roots than to infer current damage.
TABLE 3 Spearman rank coefficient correlation (ρ) matrix between root damage percentage, plant weight, months after planting and the two symphylid sampling methods used.
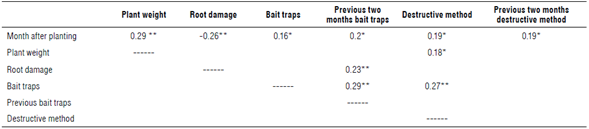
Only statistically significant values are shown. Statistically significant correlations: *P<0.05; **P<0.01.
Damage decreases significantly with the time after planting (Tab. 3). Symphylid damage and impact depend on the age of the plant and is greatest in the first months after planting (Rohrbach & Johnson, 2003). According to Saavedra (1990), symphylid damage has the greatest impact in the period of root emission that, in our case, is the first four months after planting. Roots increase their growth gradually until anthesis, when they reach their greatest number (Malézieux et al., 2003). This way, damage is compensated with an increase in radical growth six months after planting. Plants at intermediate or advanced stages of development and under good fertility conditions tolerate high populations of symphylids without affecting their growth and production (Castañeda, 1998).
The size of the plant has an influence on symphylid numbers in the destructive method (Tab. 3). Large plants may allow symphylids to shelter on the leaves that are in contact with the soil. This makes the base of the plant a more favorable environment for symphylids.
Although the correlation is significant between the two symphylid sampling methods (Tab. 3), when estimating the correlation for each sampling time, a significant correlation was found only in the months of September (p = 0.5; P = 0.009) and November (p = 0.41; P = 0.017) for the variety Perolera, while for this same variety for July (p = 0.39 and P = 0.06) it was not, nor for the three samplings in the hybrid MD2 (July p = 0.21, P = 0.27; September p = -0.01, P = 0.95; November p = 0.2, P = 0.28). Sampling in the variety Perolera for September and November showed the highest population density of symphylids from 10.8 to 11.7 symphylids per trap. When the density of symphylids was lower between 1.5 and 7.9 symphylids per trap, no correlation was found between the two sampling methods. This would indicate that the destructive method only achieves similar results as the traps when the density of symphylids is high. This result confirms the advantage of traps to detect low populations of symphylids so as to apply appropriate management strategies when populations and damage are at low levels.
Due to the greater susceptibility of the roots of seedlings at early stages of development, symphylid management practices should begin before land preparation. Sampling with baited traps would be useful to detect colonies of symphy-lids that can remain constant from year to year with little lateral displacement (Gerdeman & Diehl, 2021). To estimate the population density of symphylids, Gerdeman and Diehl (2021) recommend a sampling of at least 50 baited traps depending on the size of the lot and the time of year, but they do not recommend a specific number of traps ha-1. In our case, in most of the evaluations, an adequate estimate would be reached (except for some farms with more variability) with 75 well distributed samples. This could be an adequate minimum of samples in pineapple crops of 1 ha. Once the colonies are detected, control strategies can be targeted, reducing costs and pesticide applications.
To carry out sampling with baited traps, an estimated time of 8 h for installation and 4 h for the collection of traps is required. The inspection and extraction of symphylids required around 15 to 20 min per trap, which would indicate that a total of 19 - 25 h is needed for the 75 traps. This way, all the sampling would be carried out in 31 - 37 h (4 - 5 d) by a worker trained in recognizing symphylids. This investment in time might seem too high if the farmer does not see a tangible benefit from this effort. Although the damage of symphylids is notable and on average affects 40% of the roots, the effects on the reduction of production, delay in the life cycle, and costs of foliar fertilizers and pesticides have not been quantified, and a large percentage of farmers have not recognized symphylids as a severe problem. A research study considering these factors will be necessary to demonstrate the convenience and profitability of integrated symphylid management based on sampling with traps and appropriate and environmentally friendly management practices.
Relationship between soil pH and moisture and the presence of symphylids
The correlation between symphylids in traps and soil moisture is statistically significant (P = 0.282; P = 0.00012), confirming that percentage of soil moisture is a crucial factor in the distribution of symphylids. Although, the correlation coefficient was positive, it was expected that high soil moisture values could also decrease symphylid mobility and development (Sarah, 1990). When evaluating the preferences of soil arthropods, Ghiglieno et al. (2020) found that Symphyla is correlated with low moisture soils (<35%). In pineapple crops, the soils were in the range between 6.7% and 25%. This correlation is limited to this range of moisture. Similarly, Edwards (1961) found a significant correlation between the abundance of symphylids and soil moisture in the range between 7.5% and 15.5%.
Regarding soil pH, values were in the range from 3.1 to 5.6 that indicates strongly to extremely acid soils. No significant correlation was reported between pH and symphylids in traps (P = -0.17; P = 0.572). Umble et al. (2006) mention that the presence of the symphylid Scutigerella immaculata is not related to pH and can be found in very acidic to alkaline soils. Salazar-Moncada et al. (2015) and Ghiglieno et al. (2020) also find no relationship between the Symphyla and soil pH.
Conclusions
Symphylid sampling based on potato bait traps is superior to the practice of checking pineapple roots to sample symphylids. This methodology is easier to standardize, requires fewer samples, and is a predictor of root damage. Its incorporation into this production system can be a very useful tool for monitoring and managing symphylids.
Evaluation of symphylid monitoring based on baited traps is recommended as a decision tool in pineapple crops before planting. A number of at least 75 traps ha-1 is recommended to have an estimate of the population of symphylids.