Introduction
Colombia is the second country for fresh cut flower exports in the world (Vanegas López et al., 2017). Floriculture for exports is concentrated in six municipalities of the Sabana de Bogotá (Bogota Plateau), with production of roses, carnations, alstroemerias, gerberas, and mixed floral bouquets with sales of around 1.5 billion US dollars/year (González et al., 2014). The flower sector constitutes a non-traditional agricultural export that is based on cheap labor, fertile soil, an equatorial climate that provides around 12 h of sunshine per day throughout the year, and more than fifty years of experience (Iizuka & Gebreeyesus, 2018). This industry provides the financial and social support for workers in rural areas with nearly 200,000 employees and an important participation of women who are mainly single heads of households (Lee, 2008; Patel-Campillo, 2010).
Frankliniella occidentalis (Pergande, 1895) (Thysanoptera: Thripidae) is considered a significant pest for flower production and exports in Colombia (Loyola et al., 2019). This thrips species feeds and lays eggs directly in plant tissues causing malformation of plant structures during production, detrimental cosmetic damage for exports, transmission of virus to plants in crops that cause reduction of yield (Reitz et al., 2020) and quarantine issues for international trade (Nicholas & Follett, 2018). In Colombia, control of western flower thrips (WFT) is based on weekly insecticide applications during crop production and phosphine treatments for postharvest management (Kim et al., 2016) despite the initial development of some biological control strategies (Rueda-Ramírez et al., 2018).
For monitoring thrips populations in commercial greenhouses for exports in the Sabana de Bogotá, a direct sampling of thrips from the buds and flowers by a gentle beating of plants is carried out to determine the density of thrips per plot (relative sampling) (Pizzol et al., 2010). Additionally, plastic or acrylic yellow, white, and blue cards with insect glue are installed in the crop to determine the relative abundance and spreading of flying thrips (Corredor, 1999). In general, for standard direct plant sampling protocols in Colombia, there is no discrimination between the different species present or of population structure (larvae versus adults). This is given by the fact that, due to quarantine export regulations, no thrips should be allowed in any container (threshold = 0). In contrast, sticky cards require technical personnel for selecting colors and materials for application of glue for their use, periodic installation, counting of insects, and the incorporation of these data into entomological surveillance for thrips management strategies. These traps do not have standardized colors determined by spectral measurements or studies to define the cardinal orientation optimizing trap capture for monitoring or trapping of thrips, as is recommended by several authors (Pearsall & Myers, 2001; Ben-Yakir & Chen, 2008; Aliakbarpour & Rawi, 2011). In Colombia, information obtained from colored sticky traps is difficult to interpret by crop managers and management consultants. There are no studies showing the relationship between thrips caught on self-crafted or commercial, colored sticky traps and thrips infestation on plants and thrips damage. In general, since there are no clear criteria to incorporate data from colored sticky cards for surveillance or mass capture of thrips, they are usually abandoned by the phytosanitary programs of each floriculture company. The aim of this study was to establish the abundance and flight activity of F. occidentalis in a female chrysanthemum crop for seed production by using two different methods of direct sampling and one indirect sampling (yellow sticky traps) for thrips on plants at different phenological stages.
Materials and methods
Chrysanthemum crop conditions
This study was carried out in a female chrysanthemum crop for seed production in a plastic greenhouse located in Madrid, Cundinamarca, in the Sabana de Bogotá, Colombia (4°44' N, 74°17' W). The floor area of the greenhouse was of 1,088 m2 divided into five compartments, each separated by 0.45 m. The first area had five flower beds 0.6 m wide by 30 m length on soil substrate, and the other four areas had six flower beds of the same size. Average (18.4°C), maximum (40.3°C), and minimum (5.6°C) temperatures and relative air humidity (67.9%) were recorded every minute per day using three dataloggers distributed inside the greenhouse.
During this study, several varieties of chrysanthemum at different plant phenological stages (e.g., vegetative, flowering, senescence, and seed harvest) were present in the greenhouse at the same time, in different beds due to the production cycle. Thrips control was carried out with regular use of chemical insecticides directed at all plant phenological stages, except for those associated with the collection of pollen. The greenhouse had a main entrance with trap doors to prevent thrips from entering and moving within the greenhouse and a disinfection area for workers to get dressed and clean their shoes before entering the production area. No thrips control was done in external greenhouse areas. During the day, greenhouse temperature and relative humidity were regulated using vertical screens that could be raised and lowered between areas, but during the night, the temperature was regulated by three heaters located on the headers of areas one to three, while six fans distributed the heat. Ventilation was enabled through two longitudinal openings on the ceiling of areas one and five.
The plant material sampled for control of WFT contained plants at different stages of development: vegetative stage (VS) (1 to 8 weeks after planting), flowering stage (FS) (9 to 13 weeks after planting), senescence stage (SS) (14 to 18 weeks after planting), and seed harvest (SH) (19 weeks after planting). A grid of 454 cells (each one of 0.3 m x 0.24 m) was superimposed over the five beds to assist the selection of plants to be sampled.
Thrips plant infestation
Two direct sampling methods were used to estimate the abundance of WFT on chrysanthemum plants within the greenhouse. The relative method consisted of the gentle beating of buds and flowers (~20 sec for each plant) from a total of 240 plants (FS = 123, SS = 101, SH = 16) over a white surface (~0.15 m of diameter) where the adults and immature thrips were collected with a soft brush and preserved in 70% ethanol in plastic 1.5 ml vials labelled with the phenological stage of plants, date, and hour of collecting. The sample unit corresponded to one plant per cell (i.e., 240 plants). At the time that the relative samples were undertaken, there were no plants in the vegetative stage. The absolute method included the removal of 93 whole plants (VS = 12, FS = 51, SS = 30) from the planting beds, which were immediately immersed into 70% ethanol in plastic bags and preserved at -20°C. To protect the seeds obtained in the harvest sample (SH), plants at this phenological stage were not sampled. In the laboratory, adult thrips and immatures were counted from the ethanol solution in the sample and from the plant after careful dissection of plant material under a Nikon SMZ800® stereomicroscope (80X). Sampling was carried out in the greenhouse in March (relative method) and April (absolute method) 2018 between 9:00 and 11:00 h. Thrips were identified; 1% of the total females from direct sampling and 0.5% of females from the sticky traps were macerated and mounted on microscope slides (Mirab-Balou & Chen, 2010). Taxonomic identification was based on morphological characters of the females (Mound & Kibby, 1998; Cavalleri & Mound, 2012). The entomological material was processed at the Laboratorio de Entomología, Área Genética de Insectos de Interés Económico, Facultad de Ciencias Agrarias, Universidad Nacional de Colombia, Bogotá.
The mean number of adult and immature thrips per plant was obtained for each plant development stage. Kruskal-Wallis test (Conover & Iman, 1981) followed by Dunn's multiple comparison test (Dunn, 1964) were conducted to determine if the density of thrips changed with the plant developmental stage for each sampling method. Also, a Spearman's correlation (Puth et al., 2015) was made for the two sampling methods to assess the relationships between number of thrips and phenological stages.
Thrips flight activity
Non-commercial (self-crafted) yellow sticky tape traps (1.35 m x 0.2 m) with manually applied adhesive glue (Biotrapper®, Agrotrampas Colombiana Ltda. Bogotá, Colombia) on both faces were used to examine thrips flight activity. To examine the effect of trap height and cardinal orientation for monitoring thrips, 28 yellow sticky tape traps were placed in the greenhouse for three consecutive days in February. The bottom of each trap was placed at 0.50 m above of ground and the total height of each one was 1.85 m. Each of the yellow sticky tape traps were divided in nine squares of 0.15 m x 0.15 m, where each square (S) was represented by a different height of capture in meters from the ground (S1 = 1.85 to 1.70, S2 = 1.70 to 1.55, S3 = 1.55 to 1.40, S4 = 1.40 to 1.25, S5 = 1.25 to 1.10, S6 = 1.10 to 0.95, S7 = 0.95 to 0.80, S8 = 0.80 to 0.65, and S9 = 0.65 to 0.50). Of these 28 traps, ten yellow sticky tape traps were positioned with trapping surfaces facing the north-west/ south-east and 18 yellow sticky tapes with trapping surfaces facing the south-west/north-east. The mean number of adult thrips per trap was estimated by visual observation of the thrips on the traps and the number determined for each combination of trap height and orientation. Statistical differences were estimated using Kruskal-Wallis test (Conover & Iman, 1981) and multiple comparison of Dunn's test (Dunn, 1964). The relationships between the number of thrips vs. height and orientation of the non-commercial yellow sticky tape traps were estimated using Spearman's correlation (Puth et al., 2015).
Fifteen commercial yellow sticky traps (40 cm x 25 cm, Impact Sticky Board Yellow - Russell IPM®) were placed 7.5 m apart in the crop throughout the greenhouse. The commercial traps were inspected over a period of 10 d in March. The cumulative number of thrips per trap at different locations in the greenhouse was compared using a one-way ANOVA and a multiple comparison Duncan test (Zar, 2010). The relationships between number of thrips and the spatial location of the commercial traps were estimated using a Pearson's correlation (Obilor & Amadi, 2018).
For each commercial yellow sticky trap and non-commercial yellow sticky tape trap, the total number of thrips was counted under a Nikon SMZ800® stereomicroscope (80X) and 1% of the randomly selected samples of each trap were taxonomically identified based on morphological characters (Mound & Kibby, 1998; Cavalleri & Mound, 2012). All statistical analyses were done using the software R v 3.5.0 using a = 0.05.
Results and discussion
Thrips plant infestation
A total of 3,688 larvae, 8,513 adult females, and 325 adult males were collected. Frankliniella occidentalis (N = 7,856; 62.7%) was represented by three morphotypes based on the exoskeleton color. WFT morphotypes have been previously reported (Bryan & Smith, 1956; Elimem et al., 2011) and thought to have developed in response to changes in seasonal and environmental factors, such as temperature.
Additionally, two more species of thrips were recorded: Frankliniella sp. 1 (female N = 627; 5%) and Frankliniella sp. 2 (female N = 30; 0.2%). Since F. occidentalis was the dominant species in this study with a ratio of 40 females:1 male, it is recognized as the main pest in chrysanthemum crops for seeding (Wu et al., 2021). As there are no taxonomic keys based on morphological characters for larvae and males of thrips, these developmental insect stages were assumed to belong to WFT. For statistical analyses, only females of WFT were included.
The relative beating sampling method allowed the recovery of 1,033 larvae, 146 adult males, and 5,301 adult females of F. occidentalis, 519 females of Frankliniella sp. 1, and 22 females of Frankliniellla sp. 2 from 240 plants (29 thrips/plant). There were significant differences in thrips abundance (P<0.01) associated with the plant phenological stages with higher larvae infestation on the flowering stage (Tab. 1).
TABLE 1 Mean number (± standard error) of larvae and adult thrips collected using the relative beating sampling method at different plant phenological stages.
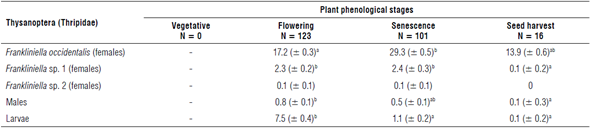
The letters represent the groups formed by the Dunn's test between stages.
The spatial distribution of larvae was not correlated with the adult distribution inside the greenhouse (Fig. 1). The highest abundance of total adult thrips was found at the senescence stage sample followed by the flowering stage (Tab. 1) with the greatest abundance of thrips located at the north end of the greenhouse (Fig. 1, large, dotted circle). Unlike the adults, the larvae were found in greater abundance in the south of the study area and in the plants at flowering stage (Fig. 1, small, dotted circles). The abundance of several thrips species is influenced by the phenological stage of plants (Cárdenas & Corredor, 1989; Mejía et al., 2018), because larvae and adults have different feeding habits, nutritional requirements, and physiological pathways to detoxify plant defenses (Kirk, 1997; Chau & Heinz, 2006; Mouden et al., 2017). Additionally, the distribution of immatures and adults can be different since once females have laid eggs on a plant, they migrate to other neighboring plants (Rhainds & Shipp, 2004).
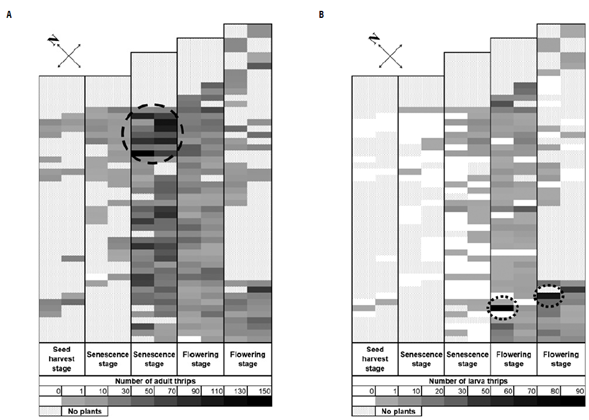
FIGURE 1 Heat map illustrating the spatial distribution of A) adults and B) larvae of thrips sampled from a range of phenological stages on a female chrysanthemum crop for seeding in a greenhouse at the Sabana de Bogotá, Colombia.
The absolute sampling method resulted in a total of 2,655 larvae, 179 adult males, and 2,555 adult females of F. occidentalis, 108 adult females of Frankliniella sp. 1, and 8 adult females of Frankliniella sp. 2 from 93 whole plants that represented an average infestation of 59 thrips per plant (Tab. 2). Larvae, female, and male infestation were found from flowering to senescence stages (P<0.01) (Tab. 2).
TABLE 2 Mean number (± standard error) of larvae and adult thrips collected using the absolute sampling method at different plant phenological stages.
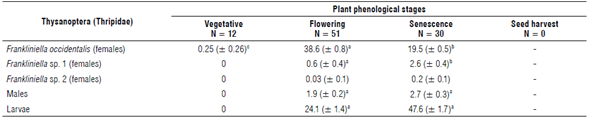
The letters represent the groups formed by the Dunn's test between stages.
No correlation (P>0.09) was found between number of thrips and those phenological stages sampled with the relative sampling method; however, a moderate negative correlation (rho = -0.4, P<0.01) was found between the number of thrips and those phenological stages sampled using the absolute method. The larvae were detected twice as much with the absolute whole plant samples compared to the relative samples (beating of flowers). The larvae may prefer to take refuge and remain in the leaves of the plant (Hansen et al., 2003), while the natural thigmotactic behavior of adult thrips allows them to escape (fly) during the removal of plants. Since the relative sampling for monitoring thrips in ornamental production in Colombia is focused on the flowering stages, the real infestation in the crop is probably underestimated (Sutherland & Parrella, 2011). Discriminating the structure of thrips populations in the greenhouse is important, not only as an indicator of resident populations that complete their full life cycle in the greenhouse (like those in this study) but also for the sensitivity of larvae to acquire and transmit orthophotospovirus to plants, where chrysanthemums are particularly susceptible to being infected (He et al., 2020; Zhao & Rosa, 2020). Because males are more efficient at transmitting viruses to plants (Ogada & Poehling, 2015), the absolute sampling that allows detection of more larvae and males is an important tool for preventing virus outbreaks in chrysanthemums.
In ornamental crops in the Sabana de Bogotá, thrips populations can reside permanently in the greenhouse because of relatively stable biotic conditions, weak plant defenses since breeders select the cosmetic attributes of the crop, and good food sources as plant fertilization is regularly applied. The genetic background of these resident thrips is improved by genes from dispersal and migrant thrips that enter through ventilation openings and main doors of the greenhouse. In this scenario, historical data on thrips plant infestation through sampling by relative and absolute methods is important to determine the structure, variation and patterns of spatial and temporal distribution of the thrips species in the greenhouse. This may help to reduce sampling costs by early detection of peak populations and sampling efforts focusing on hotspots of thrips and receptive zones for new colonization (Natwick et al., 2007). This can be done without compromising robust information for the monitoring and control of thrips, not only to reduce losses in production but also to reduce quarantine interceptions during exports.
Thrips flight activity
Non-commercial yellow sticky tape traps (self-crafted) that faced south-west caught significantly (P<0.01) more thrips (n = 9.937e) than traps facing north-west (n = 1.979a), northeast (n = 960bc), and south-east (n = 1.267*). No statistical differences were found (P>0.42) between the number of thrips versus the height for thrips caught on the vertical oriented non-commercial traps: S1 (1.85 to 1.70 m) = 1,046 thrips; S2 (1.70 to 1.55 m) = 1,224 thrips; S3 (1.55 to 1.40 m) = 1,472 thrips; S4 (1.40 to 1.25 m) = 1,427 thrips; S5 (1.25 to 1.10 m) = 1,555 thrips; S6 (1.10 to 0.95 m) = 1,715 thrips; S7 (0.95 to 0.80 m) = 1,830 thrips; S8 (0.80 to 0.65 m) = 1,988 thrips, and S9 (0.65 to 0.50 m) = 1,886 thrips. Sticky traps are generally installed at the height of the plants or a few inches above them (Bredsgaard, 1989); however, this study showed that at different heights, a high number of thrips were captured. The positive attraction response of each thrips species or thrips populations at different wavelengths using passive colors such as those on colored sticky cards and a high number of thrips infesting a crop (as in our study) can influence the effectiveness of traps to capture thrips for monitoring and mass trapping (Prema et al., 2018; Stukenberg et al., 2020). In rose crops, the blue traps at canopy level capture significantly more thrips than the yellow traps; however, the yellow traps show no significant differences for capturing thrips relating to the height from the ground (Khavand et al., 2019). A low positive correlation was found between the number of thrips vs. the height and orientation of the non-commercial traps (rho = 0.11, P = 0.01; rho = 0.24, P<0.01, respectively). This may be due to the limited sampling time used in this study. Nevertheless, the height and cardinal orientation of the traps increase the attraction of thrips species to the traps (Mao et al., 2018; Shin et al., 2020).
Commercial traps caught significantly more thrips (P<0.01) in the northern zone (N = 5,895a) of the greenhouse in comparison with the central zone (N = 4,305ab), the edge of the northern zone (N = 3,789ab), the southern zone (N = 2,681bc) and the edge of the southern zone (N = 1,116c) of the study area. A moderate positive correlation was found between number of thrips in plant samples and the spatial location of the commercial traps (rho>0.6, P<0.01).
This study showed that the trap's cardinal orientation and thrips spatial distribution within a crop and the standardized color and glue in sticky traps play an important role for the monitoring and mass trapping of thrips as an essential compound of insect pest management. Sticky colored traps allow a relative estimation of the density of adult flying thrips (Rhodes et al., 2011; Liansheng et al., 2013). This information is fundamental for developing models of spatial thrips distribution patterns using geostatistical techniques to predict dispersion of thrips inside the greenhouse; along with the abiotic conditions, these could contribute to define an early warning system for thrips management. However, colored sticky traps do not provide real information about the species and the population structure of thrips in greenhouses (Lewis, 1997) because larvae, as the biological stage for acquisition of orthotospovirus, are not represented in these (Rotenberg et al., 2015).
Frankliniella occidentalis has shown different levels of attraction to different shapes of sticky traps (Mainali & Lim, 2010), and the species is positively attracted to blue (Otieno et al., 2018; Khavand et al., 2019) and yellow colors (Roth et al., 2016). Despite this, in chrysanthemum crops in the Antioquia region of Colombia, growers use yellow sticky traps for thrips monitoring (Mejía et al., 2018). In the Sabana de Bogotá, western flower thrips also show attraction for white and purple sticky cards (Cárdenas & Corredor, 1989). The positive phototaxis of thrips to colored cards is mediated by the specific photoreceptors of the species (Matteson et al., 1992; Rőth et al., 2016) and the surface of the material and the glue (van Tol et al., 2021) that define specular or diffuse sunlight reflection (Davidson et al., 2015). The cardinal orientation of traps facing the sunlight directly or indirectly affects the reflectance of the light as an important factor for thrips response to the passive light. The architecture of the crop in its different phenological stages and external interferences, such as UV filters in plastics or nets in the greenhouse, affect thrips flight and their response to attraction cues. For example, F. occidentalis shows reduced dispersal and lower rates of attraction to yellow sticky traps under UV-deficient environments (Kigathi & Poehling, 2012). Optical response of western flower thrips populations in Colombia as an essential component of thrips monitoring and control in ornamental crops in the Sabana de Bogotá requires more studies to assess the response to different wavelengths, alone and in combination with semiochemicals (Sampson & Kirk, 2013; Abdullah et al., 2015; Cao et al., 2018; Kirk et al., 2021) and to evaluate their impact on beneficial insects for biological control of thrips and pollinators (Ogino et al., 2016; van Tol et al., 2020).
Floriculture systems in the Sabana de Bogotá are based on crops under plastic cover with different varieties and phenological stages for one or several plant species. Critical fluctuations in abiotic factors are managed through heaters, fans, and UV filters on plastic covers to protect the physiology of plants and to synchronize crop management (De Gelder et al., 2012; Shamshiri & Ismail, 2013). Density of plants and the aeration systems based on openings located in the ceiling of the greenhouse generate micro and macro environments that modulate the interaction between thrips and their hosts (Rhainds et al., 2007). All these conditions produce an ideal matrix for the establishment and maintenance of thrips populations according to their nutritional requirements, strategies to find shelters, and escape from control strategies, particularly during the spraying of chemical pesticides. Thrips management in flower crops for export from the Sabana de Bogotá is based on weekly chemical insecticide spraying. However, as the results in this research have shown, studies on thrips population dynamics, population structure, and insect-plant relationships provide a base line for implementing control strategies for thrips (Hillocks, 2002). It is necessary to design and standardize relative and absolute sampling methods to evaluate thrips management and complement the information obtained from sticky color traps. Only through these methods, it will be possible to implement control strategies for thrips that are environmentally friendly and that allow a pest and residue free production of export flowers to maintain the market in accordance with the regulations of quarantine and chemical traceability required by each country.
Conclusions
Taxonomic identification of thrips species collected by direct and indirect sampling provide the basis to develop effective integrated pest management strategies. This study has shown a resident population of F. occidentalis with high numbers of females in flowering plants and a differential spatial distribution of the larvae and adults in the greenhouse. Direct sampling of plants by relative and absolute methods provides information to determine the species and stages of development of thrips infesting plants at different phenological stages and to infer patterns of the distribution of thrips using geostatistical models. Yellow sticky traps faced south-west captured more thrips, but the height of these in the crop was not significant to increase their capture. Standardized sticky traps must be assessed in different crops and greenhouse conditions to enhance this strategy for monitoring and mass trapping thrips within an integrated pest management framework. Despite the limitations in the sampling time because the crop was eradicated by high infestation of external and resident populations of thrips, this study contributed to the knowledge of the western flower thrips populations in Colombian greenhouses and thrips monitoring in chrysanthemum crops.














