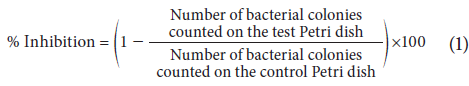Introduction
Market gardening is a vital agricultural sector in Africa because of its nutritional value and the significant economic income it generates. This sector has the capacity to serve as an impetus for agricultural and economic diversification by directing production towards local or export markets (Sadi et al., 2020).
The tomato sector in Morocco plays an important socioeconomic role. In fact, at the economic level, tomato exports have a major place since they generate about 1. 1 billion USD in foreign currency. Socially, the sector is a generator ofjobs creating an average of 9 million working days per year at the production level but also in packaging and processing (Elame, 2019).
Bacterial wilt caused by Ralstonia solanacearum is one of the major land-based vascular diseases that produces considerable damage to many crops of worldwide economic importance (Mansfield et al., 2012). The disease was discovered in Morocco for the first time on potatoes (Solanum tuberosum) in 1999 as the so-called biovar 2 (Poussier et al., 2000). Over the last 20 years, this taxonomy has undergone several revisions. The classifications were consolidated into four separate phylotypes based on the strain's geographic origin (Phylotype I, Phylotype II, Phylotype III, and Phylotype IV) (Cellier, 2010). According to Ravelomanantsoa et al. (2018), the Moroccan strain is grouped into Phylotype IIB-1 that belongs to cluster A. Penetration of this bacterium into plant tissues is ensured by wounds produced during secondary root development, insect injuries, and cultural practices. After the death of the host plant and until contact with a new host, the bacterium develops various survival strategies, such as viable non-cultivable forms and biofilm formation (EPPO, 2018). Due to the ability of R. solanacearum to develop endophytically, migrate through water, interact with weeds, and persist in soil, particularly in the deeper layers, this pathogen causes direct yield losses that vary significantly depending on the host, soil type, cultivar, cropping plan, climate, and type of strain (Elphinstone, 2005). According to some studies, bacterial wilt can result in significant yield losses in bananas (80% to 100%), potatoes (33% to 90%), tomatoes (0% to 91°%), groundnuts (0% to 20%), and tobacco (10% to 30%) (Yuliar et al., 2015).
Considering the serious consequences that pesticides may have on human health (male infertility, cancers, negative effects on fetuses, etc.), chitin and chitosan seem to be well-known and eco-friendly biocontrol agents due to their biodegradable, nontoxic, and biocompatible properties (Hassan & Chang, 2017). They act by increasing the synthesis of secondary metabolites that enhance plant immunological defenses (phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL)) (Khan et al., 2003). Recently, some studies have found that chitin and its derivatives have strong antibacterial activities in vitro and in vivo against a variety of plant pathogenic bacteria like Ralstonia solanacearum (Farag et al., 2017) and Xanthomonas spp. (Ramkissoon et al., 2016). These reactions are the consequence of the induction of multiple defensive responses in host plants, including the accumulation of phytoalexins, proteinase inhibitors, and pathogen-related (PR) proteins, callose formation and lignin production (El Hadrami et al., 2010).
This biotechnological approach to agriculture promises to increase the efficacy of agrochemicals while reducing their negative impact on the environment. Multiple evidence suggests that chitin and chitosan might be an innovative solution for the regulated release of different agrochemicals such as pesticides, micronutrients, fertilizers, and plant hormones, and they can play a dual function in plant growth promotion and biocontrol of phytopathogens (Malerba & Cerana, 2018). Foliar treatment of a commercial chitosan formulation reduced the prevalence of Xanthomonas vesicatoria in tomato plants in vivo (Li et al., 2010). Furthermore, the use of the same chitosan solution has made it possible to limit the incidence of Pseudomonas fluorescens infections on broccoli plants (Ramkissoon et al., 2016).
The aim of this study was to evaluate the efficacy of multiple treatments based on chitin and/or chitosan to protect Solanum lycopersicum L. against R. solanacearum attack.
Materials and methods
R. solanacearum isolation and biochemical identification
Sample collection
Potato tubers (variety Désirée (Red Skin)) were collected from a local market of the M'Nasra's region in the province of Kenitra, Morocco (Fig. 1). The collected samples show typical symptoms of R. solancearum infection (creamy exudates from the vascular rings and eyes of tubers).
Isolation medium
Five potato tubers were carefully cleaned with water and surface sterilized with 70% ethanol; a sterile scalpel was used to cut a transverse section. After crushing the tuber, vascular exudates were sampled with a sterile loop and streaked immediately onto SMSA medium (Siri et al., 2011), and incubated in Petri dishes at 28°C for 3 to 5 d. Colonies with the characteristic R. solanacearum phenotype (irregular shaped, fluidal, and completely white or with a pink core) were subcultured onto tetrazolium chloride agar (TZC) medium and purified for further study (Sikirou et al., 2017).
Biochemical tests for R. solanacearum identification
For validation of R. solanacearum isolates, four biochemical assays were conducted, including gram staining reaction, potassium hydroxide solubility test, the Levan test, and the sugar fermentation test (Rahman et al., 2010).
Identification of virulent and avirulent isolates
The virulent (colonies with pink or light red color or characteristic red center and whitish margin) and avirulent (smaller, off-white and non-fluidal colonies) strains of R. solanacearum were identified in a triphenyl tetrazolium chloride (TTC) medium containing 0.005% TTC (Kelman, 1954).
Virulence of the R. solanacearum strain
The virulence of R. solanacearum was assessed by inoculating tobacco (Nicotiana tabacum) plants at the six-leaf stage by infiltration, using a hypodermic syringe on the underside of the leaves. The bacterial concentration used for this purpose was of the order of 108 CFU ml-1 (DO600 = 0.28) (Adebayo & Ekpo, 2005).
The inoculated plants were then repotted in pots containing Maâmora forest soil (previously sterilized by tyndallization) (Bank et al., 2008) plus commercial peat at a rate of 1/3. According to the USDA, the soil of the Maâmora forest is made up of fine loamy sands with a clay component (De Mahieu et al., 2020). These pots were then transferred to a greenhouse at a temperature of 23 ± 2°C for 7 d.
The appearance of necrotic spots on the surface of the areas of inoculation by R. solanacearum demonstrated virulence. However, when no reaction was observed, virulence was considered negative (Schaad et al., 2001).
Source of chitin and chitosan
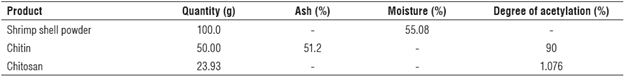
Chitin and chitosan were extracted from shrimp shells of Prapenaeus longirostris following the protocol described in previous studies (Rkhaila & Ounine, 2018). Table 1 summarizes the characteristics of these products.
Colloidal chitin preparation
Colloidal chitin was made from extracted chitin using a modified process developed by Hsu and Lockwood (1975). Forty g of chitin powder were added to 600 ml of concentrated HCl. The chitin was precipitated by the addition of 2 L of distilled water at 10°C after 2 h 30 min of stirring. The filtrate was rinsed with water until neutrality.
Chitosan solubilization
The modified technique of El Ghaouth et al. (1991) was adopted to solubilize extracted chitosan. Eighty ml of distilled water and 2.5 ml of HCl 10 N were added to 1 g of chitosan. After that, the volume was completed with distilled water up to 100 ml. Consequently, the pH of the solution was adjusted to 5.6 using 0.1 ml NaOH.
Solanum lycopersicum seed origin
The seeds used in the experiments belonged to species of the Solanaceae family from a standard variety (Rio Grande) and were homogeneous and of the same generation. The seeds are listed in the French varieties in the official catalog of the ONSSA - Morocco (Office National de Sécurité Sanitaire des Produits Alimentaires, Morocco) and authorized for sale on the Moroccan markets.
Evaluation of the effect of biopolymers on the growth of R. solanacearum in vitro
After adjusting the bacterial concentration of a 24 h culture of R. solanacearum to 108 CFU ml-1 (Rodrigues et al., 2011), 0.1 ml of this suspension was inoculated into 10 ml of Mueller Hinton broth. An aliquot of 1 ml of this well homogenized suspension was taken. Ten µm of this suspension was inoculated by streaking on Mueller Hinton agar. The plates were incubated at 26 ± 1°C for 24 h.
The liquid dilution method coupled with agar spreading was used as described by Hoekou et al. (2012) with some modifications. The assay was made by introducing into a sterile hemolysis tube 0.5 ml of the colloidal solution of chitin, chitosan, or chitin-chitosan mixture at 25, 50, or 100 mg L-1 with 10 µm of the microbial suspension. In the control tube, the extract was substituted with 0.5 ml of sterile nutrient broth. The tubes were incubated at 26 ± 1°C for 48 h; then, the tests and controls were distributed on Mueller Hinton agar medium at a rate of10 plates per tube.
After incubation at 37°C for 48 h, colonies were counted on each plate and the percentages of survival and inhibition were calculated in relation to the negative control according to Equation 1 (Hoekou et al., 2012):
In vivo test on R. solanacearum
Inoculation of S. lycopersicum seedlings
Solanum lycopersicum seedlings were grown in peat-based germination plates. At the three-leaf stage, the seedlings were collected from their substrate, and the roots cleaned with running water and lightly rubbed with cotton soaked in soapy water. Subsequently, the roots were washed until the soap was completely removed. Then, these were soaked for 30 min in various concentrations of chitin, chitosan, or chitin-chitosan mixture at 25, 50 or 100 mg L-1 with Tween 80 (0.01%) added. For control, the root system was soaked in sterile distilled water containing Tween 80 (0.01%, v/v) (Benhamou & Thériault, 1992). Finally, these seedlings were transferred to plastic pots containing Maâmora soil and commercial peat at a ratio of 1/3.
One d later, the seedlings were removed for a second time from their pots and washed with tap water. Once dried, the roots were wounded with a small diameter sterile needle to facilitate the entry of the bacteria (Chandrashekara et al., 2012). The tomato seedlings were then inoculated by soaking for 10 min in R. solanacearum bacterial suspension (108 CFU ml-1) before being replanted in their starting pots.
During the greenhouse-growing period (7 weeks), the plants were amended every week (once a week) with a 100 ml solution of chitin, chitosan or the chitin-chitosan mixture at 25, 50, or 100 mg L-1. Then, the length of the shoot/root part and the foliar alteration index were calculated.
Assessment of the degree of infection
Leaf alteration
The expression of leaf symptoms was estimated by a leaf index using the following rating scale (Douira et al., 1994):
0: leaves of healthy appearance;
1: cotyledonary leaf: wilting or yellowing of the cotyledonary leaf;
2: falling of the cotyledonary leaf;
3: wilting or yellowing of the true leaf;
4: necrosis of the true leaf;
5: fall of the true leaf.
The sum of the scores in relation to the number of leaves constitutes the leaf alteration index. An average index was then calculated for each plant (Douira & Lahlou, 1989):
where LAI is the leaf alteration index; i is the leaf appearance scores from 0 to 5; Xi is the number of leaves with i score, and NtF is the total number of leaves.
Statistical analysis
A completely randomized design was used, and data were analyzed by analysis of variance (ANOVA) using the Tukey's test at a = 0.05 and the IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp. Armonk, NY, USA). Three replicates were made for the in vitro and in vivo tests.
Biochemical tests for R. solanacearum identification
The crystal violate reaction showed that the 20 isolated strains were gram negative because they did not retain the violet color. Microscopic examination revealed that the cells of virulent isolates are gram-negative, straight, or curved rod shaped. This observation was confirmed by the appearance of a viscous mass when potassium hydroxide was applied to bacterial cultures since KOH easily dissolves the thin layer of peptidoglycan of the cell wall of gram-negative bacteria. This fact contributes to the release of its contents that generate the viscous mass (Razia et al., 2021). Furthermore, the positive result of the Levan test suggested that the R. solanacearum strains are Levan producers (Tab. 2).
For sugar fermentation, R. solanacearum isolates had the ability to oxidize carbohydrates, as shown by color changes (reddish to yellow). The sugar fermentation test revealed that all R. solanacearum strains could oxidize the four basic sugars (dextrose, sucrose, mannitol, and lactose) generating acid and gas (Tab. 2).
Identification of virulent and avirulent isolates
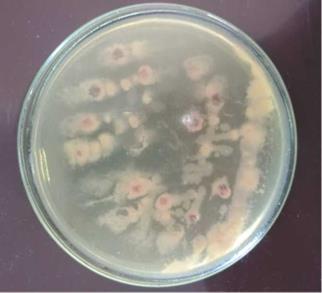
A Kelman tetrazolium chloride (CTC) agar test distinguished virulent from avirulent isolates of R. solanacearum. Additionally, in the TZC medium, these isolates generated pink or light red colonies or colonies with a distinctive red center and a white border (11 isolated strains), whereas avirulent isolates (9 isolated strains) produced tiny, off-white, and non-fluid colonies after 24 h of incubation. The results of this test showed that all R. solanacearum isolates (11 virulent strains) from the five potato tubers had gathered the typical pink or light-red colonies or colonies with a distinctive red center and a white border on the TZC medium (Ahmed et al., 2013). This proved that all isolates of R. solanacearum (11 virulent strains) were virulent (Chamedjeu et al., 2018) (Fig. 2).
Virulence of R. solanacearum
To ensure that the isolated strains were virulent, the underside of tobacco leaves was inoculated with a bacterial suspension of R. solanacearum at a concentration of 108 CFU ml-1. After incubation for 7 d, the development of necrotic spots was observed around the infiltration zones (Fig. 3). These findings are consistent with those published by Heikrujam et al. (2020), who discovered that R. solanacearum could produce a hypersensitive reaction in tobacco leaves after 7 d.
In vitro test on R. solanacearum
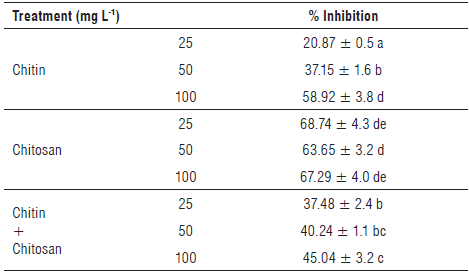
The results of the inhibition effects of treatments based on chitin, chitosan, or their mixtures on R. solanacearum are reported in Table 3.
TABLE 3 Ralstonia solanacearum growth inhibition percentage (Mueller Hinton solid media).
Means in the same column with the same letter do not differ significantly from each other at the 5% level of significance according to the Tukey's test.
From these results, all treatments resulted in the inhibition of R. solanacearum growth on Mueller Hinton agar. Furthermore, we observed a dependence between the treatment concentration and the in vitro inhibitory effect of R. solanacearum.
Based on the results of the in vitro test, we noted that all chitosan concentrations produced the highest suppression of bacterial growth, with values of 68.74%, 63.65%, and 67.29% for doses of 25, 50, and 100 mg I/1, respectively. However, the concentrations of chitin and the chitin-chitosan mixture showed a lower inhibitory effect than chitosan alone, as evidenced by inhibition percentages that did not exceed 58.92% for chitin and 45.04% for the mixture of the two biopolymers.
The study performed by Borines et al. (2015) demonstrates that chitosan caused growth inhibition of R. solanacearum in vitro, resulting in the appearance of 7 mm inhibition zones around discs imbibed with 300 mg L-1 of chitosan solubilized in acetic acid. The same authors related this effect to the high substitution of amino groups on chitosan chains, resulting in the enhancement of the positive cationic nature of chitosan in acidic solutions. This could lead to a greater chance of interaction between chitosan and the negatively charged cell walls of microorganisms (Mohy Eldin et al., 2008; Borines et al., 2015).
Whereas the results obtained by Ting et al. (2013) confirmed that an increasing chitosan concentration (from 0 to 0.15 mg ml-1) improved the inhibition of R. solanacearum with a higher rate of 74.3% obtained by applying 0.15 mg ml-1 of chitosan, analysis by transmission electron microscopy showed that this effect is the result of the destruction of the bacterial cell membrane under the action of chitosan. Thus, chitosan inhibits the formation of biofilm in many bacteria, such as Pseudomonas aeruginosa, Listeria monocytogenes, and R. solanacearum (Kim et al., 2018).
In vivo inoculation of seedlings of S. lycopersicum with R. solanacearum
Table 4 reports the results of the growth parameters and the leaf alteration index of S. lycopersicum plants inoculated with R. solanacearum.
The inoculation of S. lycopersicum seedlings strongly influenced the development of both parts (shoots and roots) in the control plants that was expressed by mean lengths varying from 36.4 ± 7.1 cm to 29.8 ± 8.0 cm, respectively. However, plants treated with the biopolymers showed an ability to overcome infection and the expression of the characteristic symptoms of bacterial wilt at both stem and root levels.
TABLE 4 Means of stem and root length (cm) and leaf alteration Index (LAI) of plants treated by chitin, chitosan or chitin-chitosan mixture (25, 50, or 100 mg L1) and control plants.
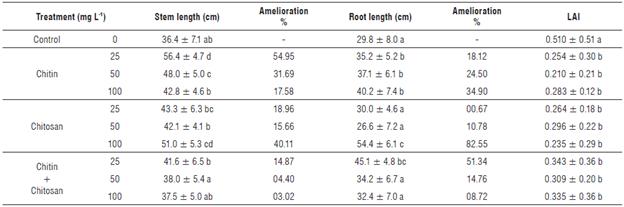
Means in the same column with the same letter do not differ significantly from each other at the 5% significance level according to the Tukey's test
An improvement of 17.58% in the growth of the shoot was recorded in plants amended with chitin at 100 mg L-1. Moreover, the treatment with 50 mg L-1 of this polymer resulted in the development of this parameter by more than 31%, while the lowest concentration (25 mg L-1) allowed for a significant increase in the growth of this part of the plants, by the expression of a difference of 54.95%.
For treatments based on chitosan, the effect of this biopolymer was strictly related to its concentration. Consequently, the increase of the concentration generated a longer shoot. Improvements of 18.96, 15.66, and 40.11% compared to the inoculated control were observed in the plants amended with the chitosan at concentrations 25, 50, or 100 mg L-1, respectively (Fig. 4).
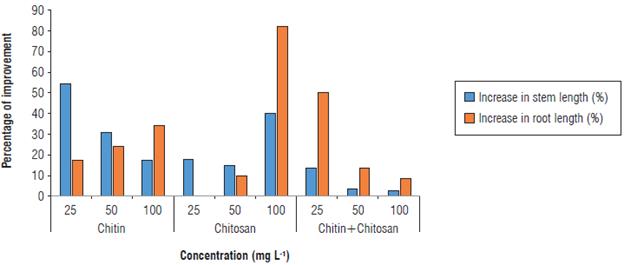
FIGURE 4 Stem and root lengths in control plants and plants inoculated with Ralstonia solanacearum, the bacterial wilt agent.
Plants whose soil was amended with the chitin-chitosan mixture at 25 mg L-1 expressed an average length of 41.6 ± 6.5 cm; in other words, an improvement of 14.87% in stem length was observed compared to the control.
Regarding the mean root length, we observed a highly significant improvement of this parameter in plants treated with chitosan at different concentrations. The strongest growth promotion was recorded by 100 mg L-1 of chitosan. This translates into a difference of 82.55% compared to the control.
Similarly, chitin at 100 mg L-1 and the chitin-chitosan mixture at 25 mg L-1 showed a remarkable increase in root length compared to the inoculated control, resulting in a difference of 34.90% and 51.34%, respectively (Fig. 4).
Seedlings amended with chitin and/or chitosan and inoculated with R. solanacearum showed the lowest LAI compared to the control. This allowed a high level of protection for the plants against bacterial wilt. Consequently, differences ranging from 48.69% to 65.05% were recorded in the presence of the chitin-chitosan mixture, while significant differences were observed when the soil was amended with chitosan (72.30% to 117.02%) or in the presence of chitin (80.24% to 142.86%). These results agree with those of Borines et al. (2015), who found that the soaking of roots in the 200 mg L-1 chitosan treatment (previously solubilized in acetic acid) before transplanting was effective in protecting the plants against the disease and, consequently, controlling bacterial wilt.
Algam et al. (2010) showed that the soil amendment with chitosan at 10 mg ml-1 significantly reduced wilt due to R. solanacearum by 72% in tomato plants grown in a greenhouse. Seed treatment with the same concentration induced a highly significant reduction of R. solanacearum incidence by 48%.
We can deduce from this that previous research employed high concentrations of biopolymers (chitin and chitosan) on the same plant; however, in our case the quantities used were lower but the results were more significant in terms of increased plant growth and bacterial biocontrol. These beneficial effects are explained by the fact that chitosan induces callóse deposition in plants (Luna et al., 2011).
Furthermore, chitin oligomers increase the synthesis of the pathogenesis related (PR) proteins (Van Loon et al., 2006) (for example, PR proteins (NPR1) in roots and leaves (PR1 and PR5) (Beatrice et al, 2017)). Chandra et al. (2015) found that chitosan treatment enhances the plant immune response by inducing defense enzyme activity, increasing phenolic contents and maximizing defense-related gene expression.
Conclusions
In this study, four different concentrations (25,50, and 100 mg L-1) of chitin, chitosan, and a chitin-chitosan mixture were employed in vitro on R. solanacearum and, subsequently, they were used preventively and curatively as soil amendments on tomato plants. The results revealed that chitosan concentrations (25, 50, and 100 mg L-1) inhibited the development of R. solanacearum more than other treatments. Furthermore, in vivo experiments revealed that the lowest concentration of chitin (25 mg L-1) allowed for excellent stem growth, whereas the same concentration of chitosan contributed significantly to root length increase. The leaf alteration index showed a decrease during the application of all treatments compared to the control. These findings led us to suggest the use of these biopolymers to manage this ubiquitous disease in matrix crops over the world.