Introduction
The common bean (Phaseolus vulgaris L.) is an essential protein source, principally for low-income farmers. The evolution of crop practices has allowed for an expressive gain in the productivity of common bean. However, there is still much subsistence farming characterized by low technology inputs, such as the application of fertilizers (Souza et al., 2013). Since the common bean is a plant with high nutritional demands, principally due to its superficial and little developed root system and short life cycle of about 90 d, the absence of fertilizer applications can have a significantly negative impact on its productivity (Oliveira et al., 2018).
Phosphorus plays an important role in all major metabolic processes in plants (Kouas et al., 2005; Khan et al., 2010). Although there is a substantial reserve of phosphorus in soils, in both inorganic and organic forms, its availability for plants is generally low, leading to a great demand for the application of P-fertilizers. However, plants can only use a small amount of this element since 75-90% of the added P becomes immobile and unavailable for plant absorption due its fixation and precipitation in the soil (Sharma et al., 2013) . It should also be considered that the P reserves are finite non-renewable sources and may run out in 50-100 years due to anthropogenic exploitation (Cordell et al., 2009). Thus, the use of this element is not fully in agreement with the principles of sustainable agriculture and, hence, environmentally friendly strategies for P utilization are required. The use of soil microorganisms with the ability to solubilize and/or mineralize soil P has been suggested as a promising tool to provide the plants with this element (Malboobi et al., 2014). Phosphate-solubilizing bacteria (PSB) metabolize phosphate by producing enzymes or low molecular weight organic acids, which increases the availability of soluble phosphate in the soil (Sadiq et al., 2013; Widdig et al., 2019).
Different bacteria can also promote the growth of plants by producing phytohormones, which are important growth regulators. For example, indoleacetic acid (IAA) is an auxin produced by several bacteria and, in the plant, regulates a large number of root biological processes (Talboys et al., 2014) . Gibberellins have a vital role in seed germination, formation of floral organs, and lateral shoot growth (Olszewski et al., 2002), while cytokinins regulate cell division and differentiation processes in the meristematic tissues of higher plants (Cassán et al., 2014). The beneficial effect on plant growth of bacteria that produce IAA, gibberellins and cytokinins has been shown in various plant species (Liu et al., 2013; Pereira & Castro, 2014).
The objective of this research was to isolate bacteria from the common bean-cultivating areas in the town of Campinas and characterize them according to their capacity to produce phytohormones and solubilize phosphate. We raised the hypothesis that bacteria with these characteristics could promote the growth of the common beans in soil with a low available P content.
Materials and methods
Isolation of bacteria
Eight bean (Phaseolus vulgaris L.) plants were uprooted along with a substantial amount of soil in a locality of the Campinas Agronomic Institute farm in the town of Campinas (22°54'20" S; 47°03'38" W), São Paulo State, Brazil. According to the Köppen classification, the climate of the region is Cfa, the mean annual temperature is 21.3°C, and the mean annual rainfall is 1462 mm.
In the field, the shoots were cut off, and the roots were placed in a container with ice and sent to the laboratory, where they were stored at 5°C for 24 h before being processed. The roots were first carefully washed with sterile distilled water to remove excess soil and then placed individually in 1 L beakers containing 500 ml of a sterile 10 mM MgSO4 solution and shaken for 60 min. An aliquot of 10 ml was removed from each beaker and added to 90 ml of sterile distilled water, from which serial dilutions up to 10-8 were prepared. From each dilution, 100 |Л were streaked over plates containing tryptic soy agar (TSA), a general-purpose medium (Atlas, 2004). Three plates were streaked for each dilution from each plant and incubated at 26°C. Microbial growth was monitored daily for about 20 d and isolated cultures were purified by streaking onto fresh agar. A total of 360 bacteria were isolated.
Selection of phosphate solubilizing bacteria
The capacity of the bacteria to solubilize phosphate was initially evaluated qualitatively by the standard dilution plating technique using the standard National Botanical Research Institute Phosphate (NBRIP) growth agar medium containing insoluble tricalcium phosphate as the sole source of insoluble P for strain growth (Nautiyal, 1999). The presence of a clear halo around the bacterial colony after 15 d of incubation, independent of its size, was used as a potential indicator of P-solubilization capacity. The bacterium was sent for a subsequent test in a liquid medium containing insoluble phosphate. The quantitative estimation of phosphate solubilization by bacteria showing halos was carried out in NBRIP medium. Portions of 25 ml of the medium contained in 125 ml Erlenmeyer flasks with a source of insoluble Ca3(PO4)2 were inoculated with 0.5 ml of the bacterial suspension adjusted to 108 cells ml-1 (optical density (OD) = 600 nm), in triplicate. The flasks were incubated at 26°C for 15 d with shaking (160 rpm) and then centrifuged at 10,000 rpm for 10 min. The soluble P concentration was determined in the supernatant using the colorimetric method (Murphy & Riley, 1962) and an autoclaved non-inoculated medium served as control.
Selection of IAA, gibberellic acid and cytokinin (zeatin) producing bacteria
To determine the phytohormone-producing capacity, the isolates were first cultivated for 24 h in 125 ml Erlenmeyer flasks containing 25 ml tryptic soy broth, and then 100 µl of each culture containing 10s cells ml-1 (OD = 600) were inoculated into 100 ml of the same culture medium contained in 250 ml Erlenmeyer flasks. The flasks were incubated at 26°C for 10 d, with shaking at 160 rpm. After this growth period, the culture media were centrifuged at 10,000 rpm for 10 min and the extract was stored at 5°C until analyzed. Phytohormones were extracted with ethyl acetate and subjected to partitioning by adding salts. After another centrifugation at 10,000 rpm for 10 min, 1 ml of the supernatant was evaporated to dryness and the residue was re-suspended in the mobile phase (75% of 0.1% formic acid and 25% of methanol). The sample was injected into an ultra-performance liquid chromatograph (UPLC) with a Waters column (Acquity UPLCâ BEH, C18; 17 µm; 2.1 x 50 mm) coupled to an electrospray ionization mass spectrometer and triple quadrupole analyzer (UPLC-ESI-MS/ MS, Quattro Premier XE, Waters, Milford, MA, USA). The total run time was 3 min. The instrument parameters were as follows: capillary (kV) 3.00; cone (V) 25.00; extractor (V) 4.00; source temperature of 120°C and desolvation temperature of 300°C. The multiple reaction monitoring (MRM) scanning mode was selected with the monitoring of two transitions per analyte. The limits of detection (LD) of the instrument were 0.01 ml-1 for cytokinins (zeatin) and IAA and 0.025 ml-1 for GA3 gibberellic acid. The limits of quantification (LQ) of the method were 0.05 µg ml-1 for zeatin, IAA, and gibberellic acid, and the analytical curve was prepared in the range from 0.05 to 1.00 µg ml-1. All the analyses were carried out in triplicate and the results for the phytohormones were given per ml of culture medium (Assalin et al., 2011).
Selection of bacteria for common bean growth promotion test in a greenhouse
Bacteria showing P solubilization values greater than 100 ml-1 were selected for the greenhouse experiment. Among these bacteria, those producing more IAA were chosen, i.e., K36, K71, T22 and A24. Bacteria producing phosphate solubilization values greater than 100 ml-1 but not producing IAA were also selected (T79 and T30). The isolate K24 was chosen for comparison since it showed low phosphate solubilizing capacity and low IAA producing capacity. The production of other phytohormones was not considered since isolates capable of producing gibberellic acid and zeatin were not found.
Greenhouse experiment
The soil used in the greenhouse experiment (Red-Yellow Oxisol) was collected in the experimental area of Environmental Embrapa in the town of Jaguariúna, São Paulo State, Brazil (22°42'20" S; 46°59'09" W). According to the Köppen classification, the climate of the region is Cfa, the mean annual temperature is 21.3°C, and the mean annual rainfall is 1462 mm.
After collecting the soil at a depth of 0-20 cm, it was air dried, finely crushed, homogenized, and passed through a 2-mm sieve. The soil chemical characteristics were evaluated according to the methodology of Camargo et al. (2009) (Tab. 1). The texture analysis obtained the following results: 41.14% clay, 12.25% silt, 30.26% coarse sand, and 16.23% fine sand.
TABLE 1 Soil chemical characteristics.
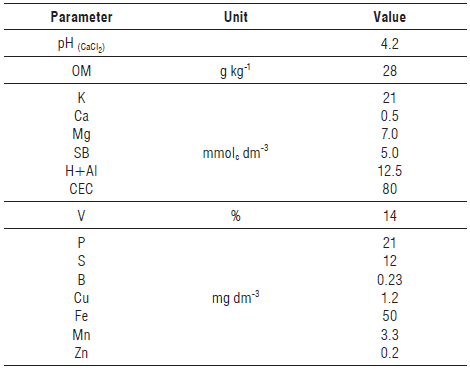
OM - organic matter; SB - sum of bases; CEC - cation exchange capacity; V - base saturation.
The experimental design was completely randomized with three replicates and the following treatments: non-inoculated bean plants (C) and bean plants inoculated separately with the bacteria K24, K36, K71, T30, T79, A24 or T22. In all treatments, the plants were cultivated with two P doses: the recommended rate (P1) and zero (P0). The non-inoculated control treatment was denominated CP when the plants were cultivated with added P, and C0 when they were not cultivated with added P. Dolomitic limestone was applied 25 d before planting at a rate of 5.2 t ha-1. For treatment P1, 30 kg P2O5 ha-1, 50 kg K2O ha-1, 20 kg S ha-1 and 3 kg Zn ha-1 were applied as simple superphosphate, potassium chloride, ferrous sulfate, and zinc sulfate, respectively. The equivalent of 70 kg N ha-1 was also added as follows: 10 kg N ha-1 at sowing and two applications of 30 kg N ha-1 on the surface 15 and 30 d after seedling emergence (DAE), in the form of urea solution. For the P0 treatment, the same applications were performed except for the phosphate application. The experiment was carried out in pots each with a capacity for 2 kg of non-sterilized soil. Four bean seeds of the variety IAC Alvorada, widely grown in Brazil, were planted per pot, but only two seedlings were left after emergence. On the day of seeding, 1 ml of each isolate, re-suspended in 10 mM MgSO4 at a concentration of 108 cells ml-1 (OD = 600), was applied to the seeds at a depth of about 1 cm. For the non-inoculated pots, the seeds only received 1 ml of 10 mM MgSO4. The crop was irrigated with water to maintain 70% field capacity. The plants were harvested 48 d after emergence and taken to the laboratory to measure the plant growth parameters, i.e., the dry weight of the shoot (DWS) and roots (DWR) and the quantities of P and N uptake by the shoot (QPUS, QNUS) (Bowman, 1989; Tedesco et al., 1995).
Data analysis
An entirely random design was used to evaluate the data on the growth promoting characteristics of the bacteria, and an analysis of variance was used to determine the probability according to the F-test. For the greenhouse experiment, the data were subjected to an analysis of variance and to the unfolding of the interaction between the factors when significant. All means were compared using the LSD test at 5% of probability. When necessary, the variables were transformed to guarantee the requisites of normality and homoscedasticity using the software R version 4.0.1. (R Core Team, 2020). Since QPUS did not guarantee these requirements, the means for this parameter were calculated after transformation of this variable using Equation 1:
Thus, lower values represented the greater P uptake by the plant.
Results and discussion
Phosphate solubilization and IAA production
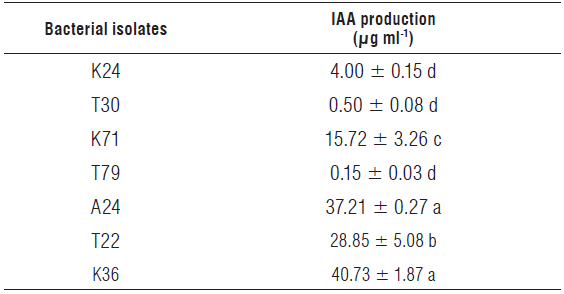
To overcome dependency on P fertilizers and reduce the production costs of bean plants, non-identified bacterial isolates with different capacities to solubilize phosphate and produce IAA were tested for their capacities to promote the growth of bean plants in soil with low available P content. The results for phosphate solubilization and IAA production were only shown for those bacteria selected for the subsequent experiment in the greenhouse. The K36, T22 and T30 isolates were those exhibiting the greatest phosphate solubilizing capacity in a liquid medium, followed by the isolates T79, K71 and A24 (Tab. 2). The isolate K24 showed a phosphate solubilizing potential 263% lower than the mean obtained for the isolates K36, T22 and T30 (206.34 ng ml-1) and 167% lower than the mean obtained for the isolates K71, T79 and A24 (151.44 µ.g ml-1). The phosphate solubilizing levels found for the isolates K36, T22 and T30 were much lower than those found by Baig et al. (2012), Lee et al. (2012), and Li et al. (2017), but similar or higher than those found by Lavakush et al. (2014). None of the bacteria produced gibberellic acid or cytokinins (zeatin) under controlled conditions but did produce IAA. Masniawaty et al. (2019) found that bacteria isolated from the rhizosphere of chili peppers can produce gibberellic acid GA3. The isolates K36 and A24 produced the maximum amounts of IAA followed by T22 and K71 (Tab. 3). The mean value found for the isolates K36 and A24 was 38.97 ml-1, whereas the mean value obtained for the isolates T22 and K71 was 22.29 ml-1. The lowest results were obtained for K24, T30 and T79 with a mean value of 1.µ55 ml-1. The production of IAA by the isolates K36 and A24 was higher than the values found by Li et al. (2017) but lower than those found by Lee et al. (2012). The results for the production of this auxin were not related to the greater capacities of the microorganisms to solubilize phosphate in a liquid medium, except for the isolate K36, and, to a lesser extent, the isolate T22.
TABLE 2 Phosphate solubilization (PS) by the bacterial isolates.
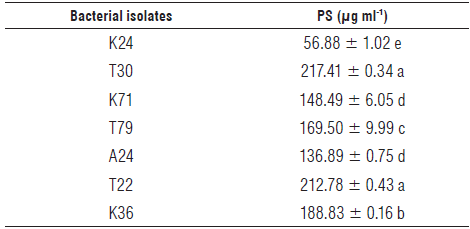
Different letters in the column differ significantly from each other according to LSD multiple range test at a significance level of 5% (P≤0.05); ± standard error.
Greenhouse experiment
Table 4 shows the values for P for the different sources of variation. Only the variable DWR was not affected by the inoculation of plants with different bacteria (P = 0.48613) or by the interaction between the factors "bacteria" and "addition of phosphate" (P = 0.16168). However, the presence or absence of phosphate fertilization significantly affected DWR (P = 0.00008), with the highest value being obtained in the treatment PI (Tab. 4).
TABLE 4 P values obtained In the analysis of variance.
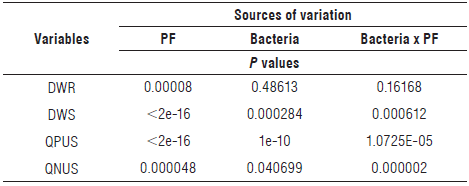
PF - Phosphate fertilizer; DWR - dry weight of the roots; DWS - dry weight of the shoot; QPUS - quantities of P uptake by the shoot; QNUS - quantities of N uptake by the shoot, e, base 10. The means for QPUS were calculated after the transformation of this variable using the equation 1 √QPUS.
The absence of a response for DWR to the inoculation of plants with different bacterial isolates (Fig. 1) could be due to the difficulty in removing the roots from the soil, which resulted in a high value for the coefficient of variation (22.93%). Despite this, the results were greater in the PI treatment.
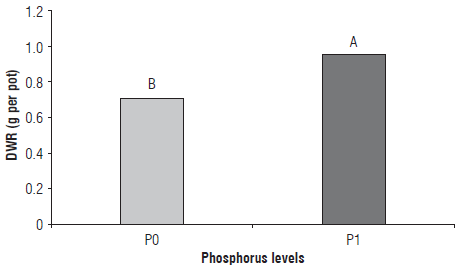
FIGURE 1 Effect of the addition of P-fertilizer on the dry weight of the roots (DWR) of common bean plants. P0: soil without added phosphate fertilizer; P1: soil with added phosphate fertilizer (30 kg P205 ha1). Different letters Indicate significant differences by the LSD test at a significance level of 5% (P≤0.05).
The DWS showed significant effects for all the factors analyzed (Fig. 2). For the treatment PO, the highest values were obtained for plants inoculated with the isolates K24 (3.95 g per pot), T79 (4.16 g per pot), T22 (4.09 g per pot) and K36 (4.03 g per pot) (Fig. 2). On average, these results were 55% higher than those of the control (CO, 2.62 g per pot).
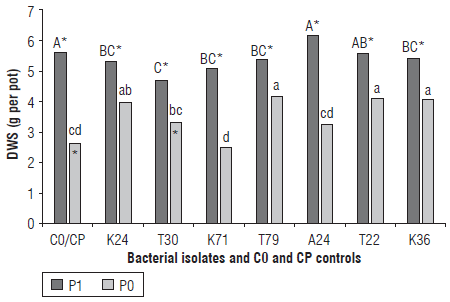
FIGURE 2 Dry weight of the shoot (DWS) of common bean plants. CO: control treatment for plants without added phosphate fertilizer; CP: control treatment for plants with added phosphate fertilizer. P0: soil without added phosphate fertilizer; P1: soil with added phosphate fertilizer (30 kg P205 ha1). Different uppercase letters In the treatment P1 or different lowercase letters in the treatment P0 differ significantly by the LSD test at a significance level of 5% (P≤0.05). *Slgniflcant differences between the treatments P0 and P1 within each Inoculation treatment.
Plants inoculated with the isolates T30 and A24 showed mean values for DWS 24.6% higher than those obtained for the treatment without the addition of phosphate fertilizer. There was no significant difference for this parameter between the plants of the control treatment (CO) and those inoculated with the isolate K71. In the treatment PI, all the values for DWS were higher than those of the treatment PO, although there were differences between the bacterial isolates for this level of P as can be seen from the higher values obtained for the control treatment (CP, 5.58 g per pot) and for the plants inoculated with the isolates A24 (6.13 g per pot) and T22 (5.54 g per pot). For the treatment PI, there were no significant differences compared to DWS for the plants inoculated with the isolates K24, K71, T79 and K36. The mean values of DWS for these bacteria were 8% lower than the means obtained for the control (CP) and for the isolates A24 and T22. The lowest values for DWS in the treatment PI were obtained for the plants inoculated with the isolate T30 (4.67 g per pot).
Due to the transformation of the data for QPUS using Equation 1, the lower values found for this parameter should be interpreted as a greater absorption of this element by the plants. In the soil without the addition of phosphate fertilizer, the lowest values found for QPUS were obtained in the plants inoculated with the isolates T79 and K36 with mean values of 0.265 mg per pot. The control treatment (CO) and the isolates T30 and T22 showed mean values of 0.31 mg per pot, the isolate K71 showed a value of 0.34 mg per pot, and the isolates K24 and A24 showed mean values of 0.365 mg per pot (Tab. 5). The mean increase in QPUS in the plants inoculated with the bacteria T79 and K36 compared to the control (C0) was 15.5%. These isolates can be considered as "real" PSB since the P plant nutrition increased directly (Bashan et al., 2013). Although the isolates T22 and T30 were the isolates that most solubilized the phosphate in the liquid culture medium, the amounts of P in the shoots did not increase, showing that the mode of action of the bacteria selected for their PS capacity is not fully understood. This agrees with other studies reporting that, although the ability to solubilize P frequently appears associated with plant growth-promoting rhizobacteria, it is not necessarily related to the ability to promote plant growth (De Freitas et al., 1997; Collavino et al., 2010). Li et al. (2017) also found that a bacterial strain did not induce the promotion of plant growth or an increase in total P despite its capacities for P solubilization and IAA production.
As expected for the soil fertilized with P, the values for QPUS were lower than those obtained in the treatment P0 for all the treatments with inoculation, although there were differences between the bacterial isolates. The lowest value obtained for QPUS in the treatment P1 was registered for the isolate K36 with a value of 0.22 mg per pot (Tab. 5). This shows that this bacterium exhibited the ability to solubilize phosphate even in the presence of phosphate fertilization. Other authors obtained bacteria with capacity to solubilize phosphate under high insoluble P conditions (De Bolle et al., 2013; Barra et al., 2019). According to Barra et al. (2019), this fact indicates that phosphorus solubilizing bacteria display an "enhancement effect" of the fertilization with triple superphosphate.
TABLE 5 Quantities of phosphorus and nitrogen uptake by the shoot (QPUS, QNUS) of common bean plants inoculated with different bacterial isolates and cultivated in soil without added phosphate fertilizer (P0) and with soil fertilized with phosphate fertilizer (P1).
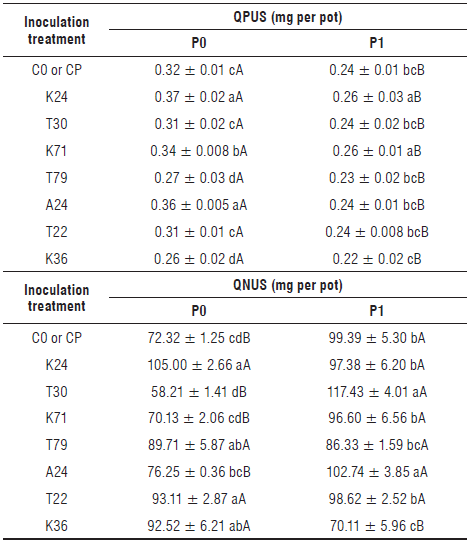
C0: control treatment for plants without added phosphate fertilizer; CP: control treatment for plants with added phosphate fertilizer. P0: soil without added phosphate fertilizer; P1: soil with added phosphate fertilizer (30 kg P2O5 ha-1). The means for QPUS were calculated after transformation of this variable using the equationl / √QPUS. Values with different lowercase letters in the column and uppercase letters in the row differ significantly from each other according to LSD multiple range test at a significance level of 5% (P≤0.05); ± standard error.
The values obtained for QNUS in the treatment P0 were higher in the plants inoculated with the isolates K24 (105.00 mg per pot), T79 (89.71 mg per pot), T22 (93.11 mg per pot), and K36 (92.52 mg per pot) (Tab. 5). These values were 45%, 24%, 29%, and 28% higher than those obtained in the C0 treatment. An increase in plant N uptake due to inoculation of the plant with phosphate solubilizing bacteria was also observed by Li et al. (2017). The results obtained for QNUS for the other isolates (T30, K71, and A24) were not significantly different from the value obtained for C0 (72.32 mg per pot). Although all QPUS values were lower in the treatment P1, this did not occur with the values obtained for QNUS as can be seen from the plant inoculated with the isolate K36, where the result for QNUS was 42% lower in the treatment P1 than that obtained in the treatment P0 (Tab. 5). In the presence of phosphorus, the highest values for QNUS were obtained for the plants inoculated with the isolates T30 (117.43 mg per pot) and A24 (102.74 mg per pot). The results for inoculation with the other isolates were significantly equal to those of the control (CP, 99.39 mg per pot). Also, regarding QNUS, there was no significant difference between the isolates K24, T79 and T22 when considering the two levels of phosphorus. The high N contents found in the plants inoculated with some of the bacteria could have been due to the ability of these microorganisms to fix atmospheric N2 or to their capacities to influence the biological fixation of N2 by the native rhizobia in a beneficial way. This last parameter was not evaluated, but all the plants showed nodules, although with reduced intensity in the control treatments (results not shown). Peix et al. (2001) reported that the bacterium Burkholderia cepacia (SAOCV2), a phosphate solubilizer, increased the growth of the bean plants and indirectly promoted nodulation, which may have led to an increase in N2 fixation.
The inoculation of bean plants with the P-solubilizing Bacillus megaterium or the N2-fixing Bacillus subtilis increased nodulation of the bean plants by the native soil Rhizobium population (Elkoca et al., 2010). However, as emphasized by some authors, an increase in the number of nodules does not always result in an increase in the N content or the dry matter of the plants, which do not depend on the number of nodules but on the efficiency of the strains (Peix et al., 2001). However, other hypotheses should also be considered since the plants received nitrogen fertilizers, which are known to interfere with the symbiotic fixation of N2 by the rhizobia (Gentili et al., 2006). Oliveira-Longatti et al. (2015) found that the inoculation of common bean with Burkholderia fungorum UFLA 04-155 and Azospirillum brasilense BR 11001T promoted nodulation by the indigenous rhizobia community in common bean plants when inoculated alone and without adding mineral nitrogen to the soil. Some authors reported that colonization by the fungus Phomopsis liquidambari could induce several genes related to N absorption and N metabolism to enhance N utilization efficiency in rice (Yang et al., 2014). Future studies are required to evaluate which processes are involved in both the growth promoting bacteria of inoculated bean plants and the increases in N uptake in soil with both low and high P content. This information could allow for a more sustainable use of nitrogen fertilizers since their production and use pose a significant environmental threat (Zhang et al., 2015).
In the treatment P0, only the plants inoculated with the isolates T79 and K36 obtained higher results for the traits to evaluate bean plant growth, as compared to the control (C0). Although the bacterium K36 was one of the isolates that most produced IAA and solubilized phosphate, this was not the case for the isolate T79. This last isolate was one of the bacteria that produced more solubilized phosphate in a liquid medium but that showed an almost non-existent capacity to produce IAA. Thus, factors other than P solu-bilization activity might have been involved. In the case of the bacterium K36, there may have been a synergistic effect between the plant growth promotion characteristics, i.e., the production of IAA and phosphate solubilization, generating greater growth and nutrient uptake in bean plants. Some authors reported that Bacillus strains with two growth promoting characteristics, i.e., P solubilization and ACC deaminase activity, were superior in improving wheat plant growth as compared to the strains possessing only a single trait (Baig et al., 2012). Bacterial isolates having beneficial traits for plants like phosphate solubilization and IAA production were capable of improving the growth of wheat when used as inoculants and, therefore, qualified for the production of wheat crop biofertilizers (Tahir et al., 2013). Since the isolate K36 increased QPUS both in the presence and absence of phosphate fertilizer, it should be tested with increasing doses of the fertilizer. A low dose of the fertilizer associated with the inoculation of that isolate could increase the productivity ofbean plants since the cost of this input is compensated in terms of yield for the small farmer. However, even using a low dose of the phosphate fertilizer, the N uptake by the plant would be affected, as observed in this study with the isolate K36. In this case, it would be pertinent to use this bacterium associated with another bacterium with the capacity to increase QNUS, such as T79 or T22, since both bacteria increased this parameter regardless of the amount of P added to the soil. Lavakush et al. (2014) reported that the combination of Pseudomonas culture (CPC) with Azotobacter chroococ-cum, Azospirillum brasilense and 30 kg P2O5 ha-1 would save 50% chemical fertilizer as compared to the treatment using a CPC with A. chroococcum, A. brasilense and 60 kg P2O5 ha-1.
Conclusions
The results of this study demonstrated the feasibility of using some phosphate-solubilizing and phytohormone-producing bacteria to increase common bean growth in soil with low available P content. The bacterial isolates T79 and K36 showed great potential for this purpose. Field tests using the isolates T79 and K36 should further elucidate their effectiveness on the grain yields of common bean plants. These bacteria are being classified for further experiments in the field.