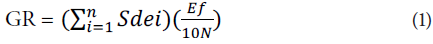Introduction
The excessive use of hazardous agrochemicals in the world to achieve high crop yields has caught the attention of the scientific community and consumers, because of the negative effects on the ecosystems and human health. The indiscriminate application of agrochemicals may increase waterway eutrophication and soil contamination with toxic heavy metals. Additionally, synthetic pesticides negatively affect biodiversity and reinforce pest resistance (Alori et al., 2017). As a viable alternative to conventional production systems, agroecological and sustainable agriculture represents a tool for reducing the use of synthetic fertilizers and pesticides to preserve the environment.
The development of new bio-agrochemicals based on soil microorganisms has emerged as a promising approach to achieve ecological management of agroecosystems (Sözer Bahadir et al., 2018). The technology of effective microorganisms (EM), also known as native or autochthonous microorganisms, consists of the inoculation of a mixed culture of beneficial microorganisms into the soil, to establish a favorable environment for crop growth and health (Olle & Williams, 2015).
Effective microorganisms promote plant growth and development in different ways. Direct mechanisms involve the production of plant hormones such as auxins, cytokinins, and gibberellins (Felestrino et al., 2017), the solubilization of minerals (Thakur et al., 2017), biological nitrogen fixation (Nushair et al., 2018), and the synthesis of iron-chelating siderophores. Indirect mechanisms include the production of antimicrobial agents and lytic enzymes that reduce populations of plant pathogens (Maan et al., 2019). Soil microorganisms are currently used to reduce the organic contaminants that result from industrial activities (Tarekegn et al., 2020) and to alleviate abiotic stresses like drought and salinity (Jochum et al., 2019).
The common bean (Phaseolus vulgaris L.) is considered one of the most cultivated and consumed dry grain legumes in developing countries and constitutes a staple food for Cuban populations. The nutritional and health benefits of this grain, rich in proteins, carbohydrates, minerals, vitamins, and dietary fiber, have been well documented (Celmeli et al., 2018; Rezende et al., 2018).
Research has focused on improving vegetative growth and yield of the common bean, using beneficial microorganisms shown to be effective in enhancing plant morphological and physiological parameters, such as number of leaves, dry weight, and yield components (Vasallo Cristia et al., 2018; Calero Hurtado et al., 2019; Calero-Hurtado et al., 2020; Gabre et al., 2020). However, the beneficial effects of EM on seed germination may also play a pivotal role in the development of the common bean and other important crops since seed inoculation with plant growth-promoting microorganisms (PGPM) leads to an improvement of vigor and seedling growth uniformity (Ayala-Villegas et al., 2014).
Additionally, seed germination is considered the most vulnerable stage in the life cycle of plants and its performance defines crop establishment and yield (Channaoui et al., 2017). Various studies have been conducted to enhance P. vulgaris germination and seedling growth by using PGPM as well as to elucidate the biochemical and physiological mechanisms by which those microorganisms stimulate seed germination (Yadav et al., 2013; Talaat et al., 2015; Kumar et al., 2016).
IHPLUS® is an EM-based product shown to be versatile for improving crop productivity and animal health and production (Blanco-Betancourt et al., 2017; Tellez-Soria & Orberá-Ratón, 2018). The hypothesis that IHPLUS® could improve germination and seedling growth of the common bean was tested under controlled conditions. The objective of this study was to evaluate the effect of IHPLUS® on germination and seedling growth of P. vulgaris L. cv. Tomeguín.
Materials and methods
Inoculum of IHPLUS®
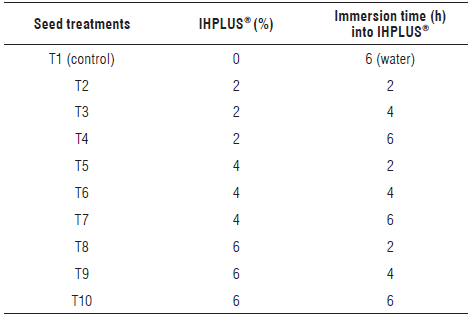
This research was developed in the Center for Biotechnology Studies of the University of Matanzas, Cuba. The germination experiment was performed under controlled laboratory conditions. The liquid inoculum of IHPLUS® was produced at the Pasture and Forage Experimental Station, Perico, Cuba and is a natural product based on native microorganisms. The composition of the main groups of beneficial microorganisms are shown in Table 1. The pH of the product was 3.45 and microbiological analysis showed no pathogenic microorganisms in the medium.
Germination test
Seeds of P. vulgaris L. cv. Tomeguín were first immersed in concentrations of IHPLUS® (2, 4, and 6%) during three immersion times (2, 4, 6 h) plus the control (distilled water) (Tab. 2). Ten seeds per Petri dish (9 cm diameter) were placed on Whatman no. 1 filter paper containing sterile distilled water in a proportion of three times the weight of the dry substrate (ISTA, 2014). Four replicates of each treatment were performed. The seeds were kept in a growing room at 25 ± 2°C with a photoperiod of 16 h (35 µmol m-2 s-1). Germination was evaluated daily for 7 d and the results were expressed as percentages of normal seedlings. Seeds were considered germinated when a radicle of at least 2 mm length emerged.
Germination rate and root and shoot length
Germination rate (GR) was determined using the formula by Djavanshir and Pourbeik (1976).
where Sdei is the speed of daily radicle emergence, calculated as the ratio between the percentage of accumulated emergence and the number of days since the beginning of the test. N is the frequency or number of Sde determined during the test, and Ef is the percentage of seedling emergence at the end of the experiment. Root and hypocotyl length (cm) were determined by using a sheet of graph paper.
Biochemical assays
Crude enzymatic extract preparation
Roots and shoots of 7-d-old seedlings were used to prepare the crude enzymatic extracts. Extracts were carried out by homogenizing plant material in a cold solution of sodium citrate buffer pH = 5.0 in a proportion of 1:2 (w/v). The homogenates were centrifuged at 10000 rpm at 4°C and the supernatants were collected and stored at -20°C for the biochemical assays.
α-amylase activity
The α-amylase activity in the seeds was determined by the procedure described by Díaz Solares et al. (2019). Enzymatic activity was expressed as a unit of α-amylase ml-1. One unit of α-amylase is defined as the amount of the enzyme that liberates 1.0 µmol of reducing sugars per min with D-glucose as a standard under the enzyme activity conditions.
Content of reducing sugars
The content of reducing sugars was determined by the method of 3.5-dinitrosalicylic acid (Miller, 1959). D-glucose (Sigma-Aldrich, Steinheim, Germany) was used as a standard, and the absorbance was measured at 456 nm.
Content of soluble proteins
Soluble protein content was determined according to Lowry et al. (1951) using bovine serum albumin (BSA) as the standard. Absorbance values were recorded at 750 nm and the concentration (mg ml-1) was calculated using a standard curve. All the spectrophotometric readings were performed in a UV/VIS spectrophotometer (Ultrospec 2000, Pharmacia Biotech, Sweden).
Experimental design and statistical analysis
The germination assay was conducted using a completely randomized design with four replicates (Petri dishes) per treatment. Five seedlings per each treatment were randomly selected to carry out the biochemical assays. Morphological parameters were evaluated by measuring 10 seedlings per treatment. Data were processed with the statistical program SPSS version 18.0 for Windows. The significance for each evaluated parameter was established by one-way analysis of variance (ANOVA) and the means were separated by the Tukey's test (P≤0.05), once the normal distribution of data and the homogeneity of variance were determined using the Shapiro-Wilk and Levene's tests, respectively.
Effect of IHPLUS® on the germination process
The results indicated that IHPLUS ® had a significant effect on the germination of P. vulgaris during the first days of this process (Fig. 1). The greatest difference among treatments was observed at d 1, when all the treatments containing IHPLUS® (except for T2) led to an increase in the percentage of germination when compared to the control. Values in those treatments ranged from 63% (T9) up to 88% (T6), with non-statistical differences, whereas T1 (Control, 30%) and T2 (43%) showed the lowest percentages without differences between them. The highest increases were observed in T6 (2.93-fold), T5 and T8 (2.66-fold) compared to the control.
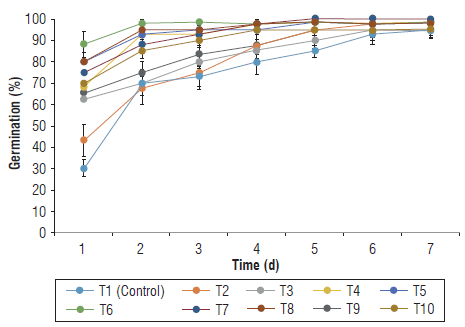
FIGURE 1 Effect of IHPLUS® on the percentage of germination of P. vulgaris L. cv. Tomeguin. T1 : Control (immersion in distilled water for 6 h); T2: 2% IHPLUS® - immersion for 2 h; T3: 2% IHPLUS® - immersion for 4 h; T4: 2% IHPLUS® - immersion for 6 h; T5: 4% IHPLUS® - immersion for 2 h; T6: 4% IHPLUS® - immersion for 4 h; T7: 4% IHPLUS® - immersion for 6 h; T8: 6% IHPLUS® - immersion for 2 h; T9: 6% IHPLUS® - immersion for 4 h, and T10: 6% IHPLUS® - immersion for 6 h. Data are the mean of four replicates and vertical bars represent the standard error (P≤0.05).
After 3 d, T6 (98%), T5 and T8 (95%), and T4 and T7 (93%) showed the highest germination percentages among the evaluated treatments, compared to the control with 73%. These results agree with other reports in P. vulgaris L. (Saxena et al., 2013) and Capsicum annuum L. (Marquina et al., 2018), where seed inoculation with PGPM also increased germination during the first days of the experiment. This is an important result since EM-based IHPLUS® seems to quickly activate seed metabolism that could be of interest in areas where water is limited.
After 7 d of the experiment, the results were similar for all the treatments and control with germination percentages above 95%. This is consistent with data reported by Wangdi et al. (2020), who obtained germination percentages higher than 95% in P. vulgaris L. after seed inoculation with beneficial microorganisms and with no differences between untreated (control) and treated seeds. This maybe associated with higher viability and seed vigor as well as suitable conditions of humidity and oxygen that ensure an efficient germination process (Finch-Savage & Bassel, 2016).
Similar studies in P. vulgaris have shown the ability of PGPM to improve the final germination percentage (Custodio et al., 2013; Yadav et al., 2013; Kumar et al., 2016; Romero-García et al., 2016). However, in those studies, control seeds showed percentages of germination lower than 75% that were suitable for improvement.
The beneficial effect of IHPLUS® on the germination of P. vulgaris might be attributed to the presence of plant growth promoter substances such as auxins, gibberellins, and cytokinins that have been reported as responsible for the growth-promoting response during embryo elongation and seedling growth of Capsicum annuum L. during the first days of germination (Marquina et al., 2018). These phytohormones are commonly produced by a large number of soil microorganisms (Taiwo et al., 2017).
Among plant hormones, the natural auxin indole 3-acetic acid (IAA) is synthetized by 80% of the soil bacteria and other microbial species (Abri et al., 2015). Recently, IAA was reportedly produced by bacillus strains isolated from IHPLUS® (Pérez-Hernández et al, 2020). Gibberellins (GAs) are involved in triggering the germination process by inducing the expression of α-amylase that hydro lyzes the starch reserve into glucose required to produce metabolic energy for embryo growth (Taiz & Zeiger, 2010). On the other hand, auxins and cytokinins act by enhancing cell division and elongation (Thakur et al., 2017).
Germination rate and seedling length
Table 3 shows the effect of IHPLUS® on the germination rate and seedling length of P. vulgaris L. The application of IHPLUS® increased the germination rate in all treatments, except for T2 that showed no difference when compared to control seedlings. The highest germination rate was observed when seeds were immersed into 4% IHPLUS® for 4h (T6) with a value of 3.39 (1.61-fold higher than control seeds immersed in distilled water at 2.11).
TABLE 3 Effect of IHPLUS® on the germination rate and seeding growth of P. vulgaris L. cv. Tomeguín.
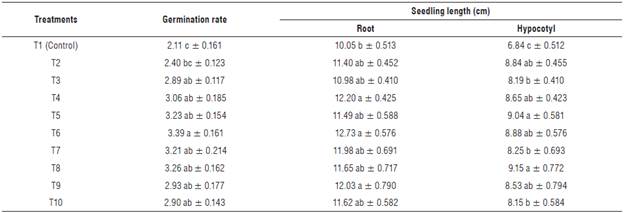
Different letters indicate statistical differences among treatments for each column according to the Tukey's test (P<0.05). T1: Control (immersion in distilled water for 6 h); T2: 2% IHPLUS® - immersion for 2 h; T3: 2% IHPLUS® - immersion for 4 h; T4: 2% IHPLUS® - immersion for 6 h; T5: 4% IHPLUS® - immersion for 2 h; T6: 4% IHPLUS® - immersion for 4 h; T7: 4% IHPLUS® -immersion for 6 h; T8: 6% IHPLUS® - immersion for 2 h; T9: 6% IHPLUS® - immersion for 4 h, and T10: 6% IHPLUS® - immersion for 6 h. Data are the mean of four replicates ± standard error.
The positive effect of beneficial microorganisms on germination rate was reported in P. vulgaris L. (Saxena et al., 2013) and other crops such as Solanum lycopersicon L. and Zea mays L. (Mahadevamurthy et al., 2016). The application of IHPLUS® also brought forward the day at which the higher number of germinated seeds was recorded (d 1), compared to the control that showed the higher germination on the second day after experimental initiation. This could be an important result, since those seeds that germinate in a short period of time show higher vigor and seedling growth uniformity (Ayala-Villegas et al, 2014). IHPLUS® promoted root and hypocotyl growth (Tab. 3). The highest increases in root length were reported in T6 (26.6%), T4 (21.4%) and T9 (19.7%), with non-statistical differences among them, but significantly higher compared to the control. The rest of the treatments showed similar results to those observed in the control. IHPLUS® significantly increased hypocotyl length in all the tested treatments, although the maximum values were recorded for T8 (33.8%), T5 (32.2%), and T6 (29.8%) when compared to control.
The growth-promoting effect of beneficial microorganisms on roots and shoots has been previously reported in P. vulgaris L. (Yadav et al, 2013; Kumar et al, 2016; Romero-García et al., 2016; Ugochi et al., 2016). IHP-LUS® was also found to increase vegetative growth of Beta vulgaris L., alone or in combination with Brevibacillus borstelensis B65 (Tellez-Soria & Orberá-Ratón, 2018). Since germinating seeds receive most of the nutrients from reserve food material available in the endosperm, the growth-promoting effect of IHPLUS® on the root and hypocotyl of P. vulgaris can be attributed to the action of phytohormones, nutrients and/or metabolites present in the IHPLUS® inoculum. Nevertheless, other experiments need to be carried out to determine the agents involved in the growth-promoting effect observed.
The recorded variations among IHPLUS® treatments may be associated with the balance among phytohormones established inside the seed tissues, after their treatment with IHPLUS® inoculum at different concentrations and times of immersion. The specific amount of endogenous and exogenous plant hormones inside the seed tissues may interact with secondary metabolites that can interfere with these hormonal pathways, resulting in a specific plant growth-promoting response. The balance between auxins and cytokinins is fundamental for plant organogenesis, and the balance shapes root architecture (Taiwo et al., 2017). Low levels of IAA can enhance primary root elongation, whereas high IAA concentration promotes the formation of lateral roots, decreases primary root length, and increases the formation of root hairs (Remans et al., 2008). Active GAs not only stimulate germination but also increase plant growth by inducing primary root elongation and lateral root extension (Yaxley et al., 2001).
Biochemical parameters
α-amylase activity
Although the beneficial effect of PGPM on the germination and vegetative growth of the common bean has been well-documented under laboratory and field conditions, little is known about the impact of single microorganisms or consortia on the amylotytic activity of P. vulgaris during germination and seedling growth. The effect of IHPLUS® on the α-amylase activity in root and hypocotyl is shown in Figure 2. The enzymatic activity in roots increased with the application of 2% IHPLUS® for 2 h (T2) and 4 h (T3) compared to control (Fig. 2). The amylolytic activity for the rest of the treatments was not significantly different between them and the control, except for T6, T9 and T10 that showed a decrease when compared to the control. The α-amylase activity in the hypocotyl significantly increased in T2, T3, T5, T9 and T10 as compared to the control. This result is consistent with other reports in Oryza sativa L., where seeds treated with biofertilizers based on rhizobacteria increased in amylolytic activity (Mohd Din et al., 2014). These authors also demonstrated that α-amylase activity is determined by several factors, such as plant genotype, the composition and concentration of the microorganisms present in the biofertilizer, as well as the physiological stage of the plant.
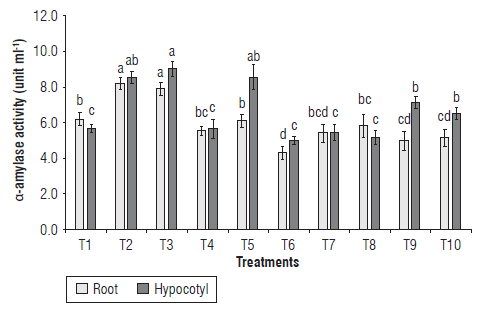
FIGURE 2 Effect of IHPLUS® on α-amylase activity of roots and shoots of P. vulgaris L. cv. Tomeguin. Different letters indicate statistical differences among the treatments according to the Tukey's test (P<0.05). T1 : Control (immersion in distilled water for 6 h); T2: 2% IHPLUS® - immersion for 2 h; T3: 2% IHPLUS® - immersion for 4 h; T4: 2% IHPLUS® - immersion for 6 h; T5: 4% IHPLUS® - immersion for 2 h; T6: 4% IHPLUS® - immersion for 4 h; T7: 4% IHPLUS® - immersion for 6 h; T8: 6% IHPLUS® - immersion for 2 h; T9: 6% IHPLUS® - immersion for 4 h, and T10: 6% IHPLUS® - immersion for 6 h. Data are the mean of four replicates and vertical bars represent the standard error (P<0.05).
The increase in α-amylase activity might result from the higher GA/abscisic acid (ABA) ratio inside the seed tissues that, in turn, depends on the endogenous GA synthetized by the embryo and the amount of exogenous GA contained in the IHPLUS® medium. Bioactive GAs induce the α-amylase gene expression and the synthesis of α-amylase that are essential to mobilize the stored starch, releasing glucose that can be used by the embryo to produce metabolic energy during respiration (Liu et al., 2018). However, other studies have reported a concentration-dependent effect of IAA on seed germination attributes and α-amylase activity (Tabatabaei et al., 2016) that suggests that those hormones, along with cytokinins, should act in concert to regulate germination.
Content of reducing sugars
The concentration of reducing sugars in roots significantly increased in T2 and T3 when compared to the untreated control (Fig. 3). The rest of the treatments showed nonsignificant differences between them and the control. IHPLUS® also enhanced the content of those compounds in the hypocotyl. The highest concentrations were shown in T2 and T3 that were 2.7-fold and 2.3-fold higher, respectively, compared to the control. The treatments T9, TIO, T7 and T5 also increased the content of reducing sugars compared to the control seedlings.
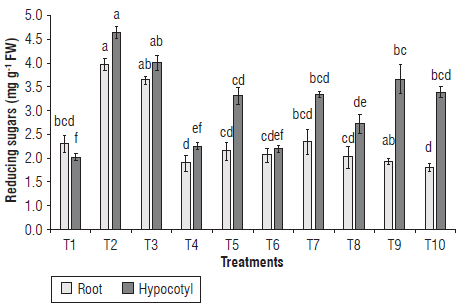
FIGURE 3 Effect of IHPLUS® on the content of reducing sugars in roots and hypocotyls of P. vulgaris L. cv. Tomeguin. Different letters indicate statistical differences among the treatments according to the Tukey's test (P<0.05). T1 : Control (immersion in distilled water for 6 h); T2: 2% IHPLUS® - immersion for 2 h; T3: 2% IHPLUS® - immersion for 4 h; T4: 2% IHPLUS® - immersion for 6 h; T5: 4% IHPLUS® - immersion for 2 h; T6: 4% IHPLUS® - immersion for 4 h; T7: 4% IHPLUS® - immersion for 6 h; T8: 6% IHPLUS® - immersion for 2 h; T9: 6% IHPLUS® - immersion for 4 h, and T10: 6% IHPLUS® - immersion for 6 h. Data are the mean of four replicates and vertical bars represent the standard error (P<0.05).
Such an increment in reducing sugars might be attributed to the increase in α-amylase activity observed in the tested seedlings. The molecules of glucose released by the catalytic activity of α-amylase are essential to obtain metabolic energy through the respiration process and represent a source of carbon skeletons to form new molecules and cellular structures in growing tissues and organs (Taiz & Zeiger, 2010).
The results agree with those reported by Angeles-Núñez et al. (2015) in plantlets of P. vulgaris L. var. Flor de Mayo, treated with Ramlibacter sp., Sinorhizobium sp., Sinorhizobium fredii and Bradyrhizobium japonicum. In that study, an increase in reducing sugar content (glucose and fructose) in roots was reported as compared to the control. However, only Ramlibacter sp. was able to increase the reducing sugars in shoots, demonstrating the importance of the plant-microorganism interaction on the plant physiological response. Those authors also explained the change in soluble sugars due to an increase in α-amylase activity associated with raising bioactive GAs.
Total soluble proteins
The application of IHPLUS® to seeds of common bean increased protein contents in the root and hypocotyl of 7-d-old seedlings (Fig. 4). The highest protein content in the root was observed in seedlings treated with 2% IHPLUS® and 4 h of immersion (T3), followed by T2, T5, and T8, with non-significant differences among them, but significantly higher values compared to the control. The protein content in the hypocotyl increased in all treatments with IHPLUS®, except for T6, T9 and T10 that showed similar values to the control seedlings. The highest contents were recorded in seedlings treated with T3 and T2, with 5.3-fold and 3.4-fold increases, respectively, compared to the control. Similar results were reported by Prathibha and Siddalingeshwara (2013) in Sorghum bicolor L. varieties CSH-14 and Proagro, who found an increase in total protein content after the seed inoculation with Pseudomonas fluorescens and Bacillus subtilis.
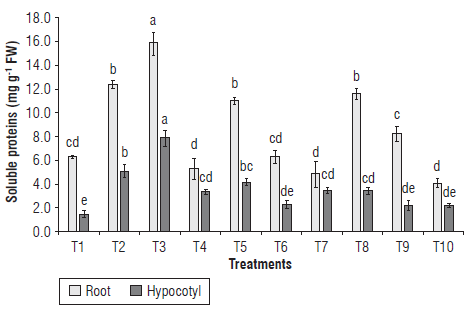
FIGURE 4 Effect of IHPLUS® on the content of soluble proteins In seedlings of R vulgaris L. cv. Tomeguin. Different letters indicate statistical differences among the treatments according to the Tukey's test (P<0.05). T1: Control (immersion in distilled water for 6 h); T2: 2% IHPLUS® - immersion for 2 h; T3: 2% IHPLUS® - immersion for 4 h; T4: 2% IHPLUS® - immersion for 6 h; T5: 4% IHPLUS® - immersion for 2 h; T6: 4% IHPLUS® - immersion for 4 h; T7: 4% IHPLUS® - immersion for 6 h; T8: 6% IHPLUS® - immersion for 2 h; T9: 6% IHPLUS® - immersion for 4 h, and T10: 6% IHPLUS® - immersion for 6 h. Data are the mean of four replicates and vertical bars represent the standard error (P≤0.05).
The stimulating effect of IHPLUS® on protein metabolism maybe partially associated with the availability of reducing sugars found in the seedling tissues. Reducing sugars may be used not only for obtaining metabolic energy during cellular respiration, but also for supplying carbon skeletons to form amino acids and other molecules after their oxidation during glycolysis and the tricarboxylic acid cycle (Taiz & Zeiger, 2010). Thus, the growth-promoting effect of IHPLUS® might enhance the protein biosynthesis that is essential to support the functioning of the new growing seedling tissues and organs.
The results obtained here demonstrate the capability of IHPLUS® to enhance P. vulgaris germination, seedling growth, and metabolism that is essential for latter seedling establishment and crop yield (Channaoui et al., 2017; Ha-Tran et al., 2021). Therefore, IHPLUS® might be considered an eco-friendly alternative to hazardous chemical fertilizers to promote seed germination and seedling growth of common beans.
In general, the immersion of P. vulgaris seeds into IHPLUS® enhanced their metabolism by increasing α-amylase activity; however, other enzymatic pathways different to this might be stimulated since plant growth promoting microorganisms may elicit distinct phytohormone-related pathways simultaneously, conferring additive responses that enhance seedling growth and development (Gholami et al, 2009).
The metabolic energy derived from the oxidation of reducing sugars is an essential factor for the active biosynthetic processes that take place in the embryo tissues during germination. It also supports the energy demand for cell division and elongation that is intense in the first stages of plant development and lead to vigorous seed germination (Yan et al, 2014).
Considering all the obtained results, it was possible to select those treatments with the highest impact on seed germination and seedling growth of P. vulgaris. Among them, T5 (4% IHPLUS® - immersion for 2 h) and T6 (4% IHPLUS® - immersion for 4 h) resulted in the best combinations of dose/immersion times. These treatments were able to increase the germination percentage in a short period of time, the germination rate, as well as root and hypocotyl length. Root growth promotion is particularly important to achieve a large root system as quickly as possible that allows the plant to explore soil more efficiently and find nutrients to sustain growth. Similarly, T8 (6% IHPLUS® - immersion for 2h) was shown to be effective for stimulating seed germination and seedling growth; however, from an economical point of view, T5 and T6 seem to be more suitable candidates.
Conclusions
The application of EM-based IHPLUS® to seeds of P. vulgaris induced biochemical changes in the seedlings that led to improvement in seed germination and seedling growth. IHPLUS® improved the germination percentage in the first days of the experiment and the germination rate that is important to obtain rapid germination and seedling growth uniformity, suitable qualities for achieving higher crop yield. IHPLUS® also increased root length that may ensure a rapid anchorage of the plantlet to soil and nutrient uptake. Among the assessed treatments, T5 and T6 showed the most advantageous results from the biological and economical points of view.