Introduction
Potassium (K) is important for cellular processes, such as cell turgor and elongation, and quality factors such as organoleptic and aesthetic properties of the plants; however, these factors do not represent direct contributions to crop yields (Sardans & Peñuelas, 2015). For this reason, the importance of the soil as a supplier of K is less in extensive field crops, such as cotton or cereals, which are not paid for quality but for quantity. In these cases, many farmers neglect K fertilization generally in favor of nitrogen (N) causing a nutritional imbalance, leading to a decrease in K reserves in the soil, low yields and increased production costs (Shirale et al., 2019; Kour, Rana, Kaur, et al., 2020).
Potassium in soil represents 2.6% of the weight of the Earth's crust and can be found in two fundamental forms, as free ions in the soil solution and in diverse forms in different mineral and organic compounds. Despite this abundance, 90-98% of K is fixed in forms not available to plants (Sun et al., 2020) within the minerals of magmatic origin such as potassium feldspars and micas such as muscovite and biotite (Shirale et al., 2019). The low amounts of available K in the soil tend to deplete quickly during cultivation due to high demand by some plants. Therefore, the constant renewal of easily removable K sources is required, such as from mineral fertilizers that represent around 95% of the K exploited in the world (Shirale et al., 2019).
The release of K from minerals occurs slowly through weathering or by the action of a diverse group of microorganisms including fungi and bacteria (Sarikhani et al., 2018); bacteria play an essential role in the biogeochemical cycle of this element (Sattar et al., 2019). There is little information related to the microbial process of K solubilization at the molecular level. However, existing reports indicate that the K release mechanisms used by these microbes include: a) a direct solubilization pathway (strong organic acid production) (Basak et al., 2017; Sattar et al., 2019); b) an indirect solubilization pathway (chelation of cations bound to K silicate, exchange or complexation reactions, and metal complexing ligands) (Basak et al., 2017), c) secretion of polysaccharides (capsular exopolysaccharides) (Sattar et al., 2019), and d) biofilm formation on mineral surfaces (Nagaraju et al., 2017). The exudation of organic acids, especially oxalic, tartaric, and citric (Rajawat et al., 2016), may be the most important mechanism of solubilization of K minerals (Etesami et al., 2017), mainly muscovite, feldspar and biotite (Basak et al., 2017).
Previous studies have focused on potassium solubilizing bacteria (KSB), especially those that inhabit the rhizo-spheric zone, where genera such as Pseudomonas, Pantoea, Rahnella, Bacillus, and Paenibacillus have been found (Xiao et al., 2017; Khanghahi et al., 2018; Dong et al., 2019). Inoculation of KSB in crops of agricultural interest like tea, rice, wheat, barley, tomato, lettuce, cotton and corn has shown good results in growth variables such as plant height, length and dry weight of leaves and roots, shoot growth, biomass yield, nutrient uptake, and fruit weight and yield (Bagyalakshmi et al., 2017; Khanghahi et al., 2018; Sarikhani et al., 2018; Sattar et al., 2019), in addition to a notable increase in the available forms of K in the rhizosphere and in the K uptake by plants. However, most of these studies use bacteria isolated from ecosystems or crops different from those where they are inoculated. In this sense, the use of native strains resident in the rhizosphere of the crop may represent an advantage compared to the use of allochthonous strains. The autochthonous strains exhibit better capacity to interact positively with the resident edaphic microbiota, greater adaptability to local climatic and agroecological conditions, and the promotion of sustainable ecological management of agroecosystems (Reinhold-Hurek et al., 2015; Koskey et al., 2017; Berger et al., 2018; Tchakounté et al., 2018).
Corn is an exhaustive crop whose nutritional needs include N and K to a greater extent (Feng et al., 2019); sometimes, the K requirement is higher than the N requirement depending on the chemical characteristics of the soil (Wang et al., 2018). This leads to the use of fertilizers as a common and mandatory practice to obtain acceptable yields in this crop. However, the long-term indiscriminate use of agrochemicals has a negative impact on agricultural productivity (Wang et al., 2018; Kour, Rana, Kaur, et al., 2020). Furthermore, it constitutes the main cause of loss of soil fertility (Berger et al., 2018) and generates groundwater contamination (Sun et al., 2020), the displacement of microbial communities or the loss of some of their ecological functions, and, in some cases, affectations of human health (Vejan et al., 2016). In Cuba, the land area destined to the cultivation of corn is greater compared to other crops of agricultural interest, with a total of 128,604 ha in 2020 (ONEI, 2021). However, the crop yield is remarkably low (2 t ha-1) and does not meet the nutritional demands of the population or the livestock sector (ONEI, 2021). Fundora et al. (2010) state that this is due to insufficiencies in the production chain, fundamentally to the shortage of chemical fertilizers (N and K fertilizers), which represents the highest cost of the process. Additionally, nutrient deficiencies and the deterioration of the structure of Cuban soils as a result of long years of monoculture and intensive cultivation threaten the agricultural sustainability and yield of this crop. For this reason, the aim of this research was to identify and characterize KSB from corn rhizoplane for future use as inoculants for this crop.
Materials and methods
Sampling
Sampling was carried out at the "El Mulato" farm (23°00'26.8" N; 82°08'12.6" W), Mayabeque province, Cuba. Thirty-day old (vegetative development) corn plants grown in leached Red Ferralitic soil were sampled. Five random sampling points were established, and two plants were selected from each of them. A 20 cm3 region was excavated around the root system, which was completely extracted. A sample of non-rhizospheric soil and rhizospheric soil was collected at each of the sampling points for chemical characterization in the Chemical Analysis Laboratory of the National Institute of Agricultural Sciences. The root samples were placed in polyethylene bags and kept at 4°C until their processing in the Microbiology Laboratory of the same center.
Chemical characterization of the soil
The pH of the rhizospheric and non-rhizospheric soil samples was measured by potentiometry in a 1:2.5 (1N) soil/KCl suspension. Moisture content was determined gravimetrically by drying a sample of wet field soil in an oven at 105°C for 24 h. The organic matter content was determined by the wet combustion method with potassium dichromate (K2Cr2O7) (1N) (Paneque Pérez et al., 2010). The total K was extracted from the air-dried soil by digestion in a hot concentrated mixture of 7 ml of HNO3 and 1 ml of HClO4, held for 3 h and then adjusted to 100 ml after cooling, while the available K was extracted from 1 M NH4OAc (Sun et al., 2020). Both extraction solutions were analyzed by flame emission spectrophotometry (X = 766 nm) (410 Classic Flame Photometer, Sherwood Scientific Ltd, UK).
Isolation of potassium solubilizing bacteria
The isolation of potassium solubilizing bacteria was carried out from the rhizoplane of the corn plants on solid Aleksandrov culture medium (5 g L-1 glucose, 0.005 g L-1 MgSO4.7H2O, 0.1 g L-1 FeCl3, 2.0 g L-1 CaCO3, 3.0 g L-1 K-feldspar powder, 2.0 g L-1 Ca3(PO4)2, 20 g L-1 agar, pH 7.5) (Vincent, 1970). First, the roots were superficially washed with sterile water until most of the attached rhizospheric soil was removed. Clean roots were then cut into 1 cm long portions and placed in 50 ml Erlenmeyer flasks with 10 ml of sterile distilled water. The flasks were continuously stirred for 2 h at 150 rpm. With the suspensions obtained, serial decimal dilutions from 10-1 to 10-6 were made, of which the 10-4, 10-5 and 10-6 dilutions were cultured by dissemination on solid Aleksandrov medium. Potassium feldspar powder (K-feldspar) was used as the insoluble mineral. The cultures were incubated at 29 ± 1°C for 7 d. The colonies that formed a solubilization halo around themselves were selected and re-isolated on Aleksandrov medium under the same culture conditions by successive passages. Once the axenic cultures were obtained, they were stored at 4°C for future use.
Morphological and colony characterization
For the colony characterization, the coloration, opacity, shape, elevation, edges, and mucus of the colonies on Aleksandrov solid medium were considered. A Gram stain was performed to determine morphology, staining, and sporulation.
Identification and phylogenetic analysis
The molecular identification of KSB was carried out by partial sequencing of the 16S rDNA. The extraction of the genetic material was carried out by alkaline lysis of bacterial colonies in Eppendorf tubes containing 40µL of NaOH (0.2 M). The suspension was heated in a microwave oven at 10% power for 1 min and then immediately cooled on ice for 5 min. Subsequently, the suspension was centrifuged for 10 min at 10,000 g (Eppendorf 5702R, Merck KGaA, Germany) to remove cell debris and the supernatant was stored at room temperature.
Amplification of 16S rDNA was carried out by the PCR technique, using a 25 µL mixture containing 1 µL (10 µM) of universal primers 27F (5'-AGAGTTTGATCCTGGCT-CAG-3 ') and 1492R (5'-TACGGTTACCTTGTTACGACTT -3 '), 1 µL of rDNA, and 23 µL of GoTaq® Green Master Mix (Promega Corporation, USA). The process was carried out in an MS mini thermal cycler (Major Science, USA) with the following parameters: initial denaturation 95°C for 10 min and additional denaturation 92°C for 1 min; 35 cycles at 72°C each for 2 min. The amplification products were observed by 1% (m/v) agarose gel electrophoresis (1 g of agarose in 150 ml of 1X TAE buffer) at 80 V for 45 min. Sequencing was performed using the Sanger method at the Macrogen® company (Republic of Korea, http://www.macrogen.com/en/main/index.php).
Subsequently, the obtained sequences were indexed and compared with the National Center for Biotechnology Information (NCBI) database using the BLASTn tool (basic local alignment search tool) and aligned using the ClustalW multiple sequence alignment tool in the MEGA-X software (version 10.0.4) (Kumar et al., 2018). Additionally, a phylogenetic tree was made from the sequences obtained and the closest type sequences using the Neighbor-joining method in the same software.
K solubilizing capacity
Bacterial suspensions of each isolate were prepared in sterile distilled water and the optical density was adjusted to 0.5 (λ = 600 nm) by spectrophotometry (Thermo GENESYS™ 10UV UV-Vis, Thermo Fisher Scientific, USA).
The solubilizing capacity was determined by calculating the solubilization index (SI) on solid Aleksandrov medium (30°C, pH 7) using two separate insoluble sources of K, K-feldspar (3 g L-1) and muscovite (3 g L-1). Ten µL of the bacterial suspensions were inoculated for a total of four isolates per plate, and six replicates were established per isolate. After 7 d of incubation, the SI was calculated using the formula:
where DH is the diameter of the solubilization halo (cm) and DC is the diameter of the bacterial colony (cm).
The dissolution rate was characterized as "fast" when the solubilization halo appeared before the third day of culture and "slow" if it did so later. The solubilizing capacity was characterized as "low" (SI<2.00), "intermediate" (2.00≤SI≤4.00) and "high" (SI≥4.00) according to Saha et al. (2016).
K releasing capacity under different conditions of temperature, pH, and salinity
One hundred milliliter Erlenmeyer flasks were prepared with 20 ml of the liquid Aleksandrov medium independently supplemented with K-feldspar (3 g L-1) and muscovite (3 g L-1). To evaluate the effect of pH, the medium was adjusted to values of 5.5, 7.5, and 9.0 by adding hydrochloric acid (HCl) or sodium hydroxide (NaOH). Four ranges of salinity were established by applying 4 g L-1, 8 g L-1, 12 g L-1, and 16 g L-1 of sodium chloride (NaCl) and the effect of temperature was verified at 28°C, 30°C, 37°C, and 40°C. In all cases, the Erlenmeyer flasks were continuously stirred at 150 rpm for 7 d. The cultures were subsequently centrifuged at 3000 g (Eppendorf 58XX with rotor F-34-6-38, USA) for 15 min and the amount of K released in the supernatant was determined by flame spectrophotometry (BWB SFP Flame Photometer, BWB Technology, UK). The standard curve was prepared with a potassium chloride (KCl) solution (Saha et al., 2016). Six replicates were established per treatment.
Statistical analysis
The data from the determinations of the solubilizing and releasing capacity of K from K-feldspar and muscovite were subjected to the normality test (Bartlett's test) and homogeneity of variance (Kolmogorov-Smirnov's test). Simple classification analysis of variance (ANOVA) was applied, using the Tukey's mean comparison test at P<0.05. Version 21 of the statistical package for the social sciences program (SPSS®, IBM Corporation, USA) was used for the statistical processing of the data, and Excel 2016 to create the graphs.
Results
Chemical characterization of the soil
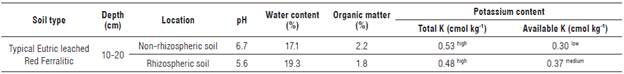
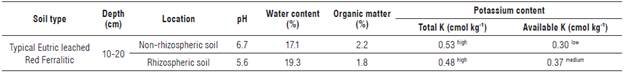
The pH at the rhizosphere level was slightly more acidic compared to the non-rhizospheric soil, which was found closer to neutral. The water content indicated good moisture retention, while the organic matter levels were low. The total K content determined in the non-rhizospheric soil was higher compared to the rhizospheric soil, although both values were considered relatively high according to John-Louis et al. (2017). However, the available K values for the plants were considered low in non-rhizospheric soil and medium in rhizospheric soil (Tab. 1) (John-Louis et al., 2017).
Isolation and morphological characterization
A total of eight bacterial isolates with the presence of a solubilization halo around the colonies were obtained on the solid Aleksandrov culture medium. The cultural and morphological characterization showed diversity among the selected isolates (Tab. 2). Five of these were characterized as Gram-positive and three as Gram-negative. The INCA-FRr15 and INCA-FRr5 isolates showed identical morphological and colony characteristics.
Identification and phylogenetic analysis
The partial sequencing of the 16S rDNA of the eight isolates allowed the identification of six genera (Tab. 3) corresponding to the phyla Actinobacteria, Firmicutes and Proteobacteria (Fig. 1).
TABLE 3 Molecular identification of potassium solubilizing bacteria (KSB) from corn rhizoplane, from the partial sequencing of 16S rDNA.
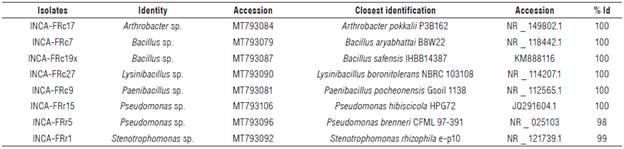
% Id - Percentage of identity.
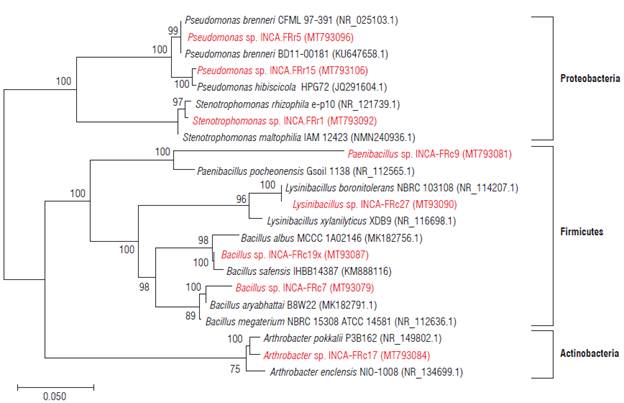
FIGURE 1 Phylogenetic tree obtained by the Neighbor-joining method with 1000 bootstrap replicas using partial 16S rDNA sequences belonging to 22 strains. The numbers in each branch correspond to the percentage of replicated trees that are associated with the taxon in question and the evolutionary distances were determined by the maximum likelihood method. The potassium solubilizing bacteria (KSB) strains identified in this study are shown in red.
К solubilizing capacity
One hundred percent of the strains developed a solubilization halo both on the medium supplemented with K-feldspar and on the medium supplemented with muscovite. Additionally, the appearance of the halo occurred within the first 48 h of the culture, so the dissolution rate was classified as fast.
The solubilization indices remained within the ranges of 0.73 - 5.36 for K-feldspar and 1.07 - 3.39 for muscovite. The solubilizing capacity did not vary from one mineral to another except for the strains Bacillus sp. INCA-FRc7 and Bacillus sp. INCA-FRcl9x, which showed contrasting solubilizing capacities, high for K-feldspar mineral and low for muscovite. In the case of К solubilization from muscovite, the most prominent strain was Arthrobacter sp. INCA-FRcl7, with an SI of 3.39 for an intermediate solubilizing capacity (Tab. 4).
TABLE 4 Determination of the solubilization index (SI) and solubilizing capacity (SC) of K-feldspar and muscovite on solid Aleksandrov medium (30°C, pH 7).
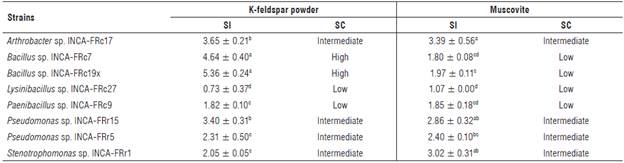
Tukey's mean comparison test (P<0.05; n = 6). Values represent means ± standard error. Different letters in the same column indicate statistical differences.
К releasing capacity under different conditions of temperature, pH, and salinity
In general, the Gram-positive strains performed better under the different cultivation conditions evaluated, except for Lysinibacillus sp. INCA-FRc27 and Paenibacillus sp. INCA-FRc9, which showed the lowest values in all determinations. Similarly, the amounts of К released from muscovite were higher compared to those obtained from K-feldspar (Figs. 2-4).
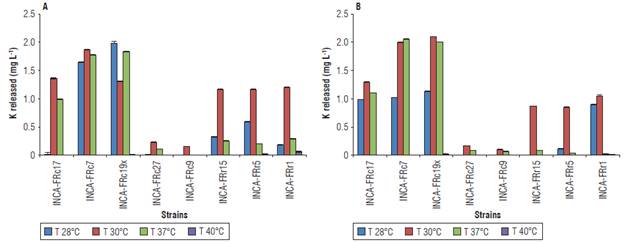
FIGURE 2 К released from A) K-feldspar powder and B) muscovite in liquid Aleksandrov medium under different conditions of temperature. Values represent means ± standard deviation (n = 5).
The temperature that most favored the release of К in both sources was 30°C (Fig. 2). The most efficient strains were Bacillus sp. INCA-FRcl9x with 1.982 mg L-1 of К released from K-feldspar at 28°C and 2.096 mg L-1 from muscovite at 30°C, and Bacillus sp. INCA-FRc7 with 1.873 mg L-1 of К released from K-feldspar at 30°C and 2.051 mg L-1 from muscovite at 37°C. The Gram-negative strains obtained similar amounts of К released in each source of 1.162 - 1.201 mg L-1 in K-feldspar (Fig. 2B) and 0.850 - 1.044 mg L-1 in muscovite at 30°C (Fig. 2B). The strain Stenotrophomonas sp. INCA-FRrl was the only one to release detectable amounts of К from K-feldspar at all four temperatures (Fig. 2A).
К solubilizing activity was favored at pH 7.5, where all the strains, except for Pseudomonas sp. INCA-FRr5, released between 0.490 - 2.001 mg L-1 (Fig. 3). The highest values with this pH were obtained by the strains Bacillus sp. INCA-FRcl9x and Bacillus sp. INCA-FRc7, which reached up to 1.798 mg L-1 from K-feldspar (Fig. ЗА) and 1.986 mg L-1 from muscovite (Fig. 3B). However, the highest values of К released were obtained at pH 5.5 by these same strains.
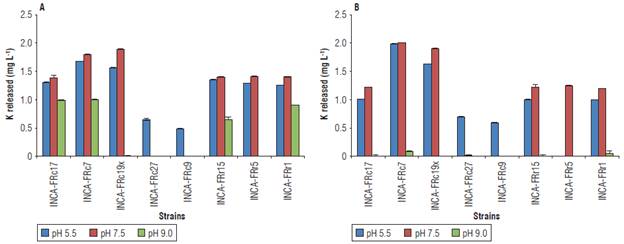
FIGURE 3 К released from A) K-feldspar powder and B) muscovite In liquid Aleksandrov medium under different conditions of pH. Values represent means ± standard deviation (n = 5).
On the other hand, the alkaline pH was more successful in releasing К from K-feldspar, with the highest value for the strain Bacillus sp. INCA-FRc7 with 1.004 mg L-1. Strains Arthrobacter sp. INCA-FRcl7, Bacillus sp. INCA-FRc7, Pseudomonas sp. INCA-FRrl5 and Stenotrophomonas sp. INCA-FRrl released detectable amounts of К from K-feldspar under the three measured pH conditions (Fig. ЗА), while Bacillus sp. INCA-FRc7 and Stenotrophomonas sp. INCA-FRrl released it from muscovite (Fig. 3B).
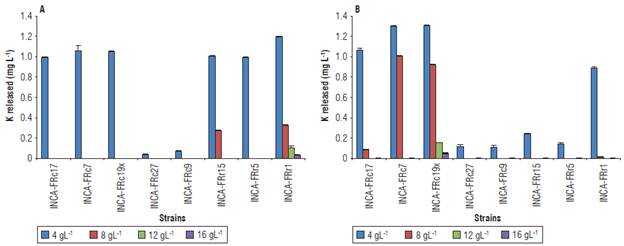
Salinity affected the release of K, mainly at high concentrations of NaCl (Fig. 4). The К releasing activity was manifested in all strains at a concentration of 4 g L-1 of salt, with 0.036 - 1.306 mg L-1 released from K-feldspar and muscovite. The highest values of К released were obtained at this concentration by the strain Stenotrophomonas sp. INCA-FRr1 from К-feldspar (Fig. 4A) and by strains Bacillus sp. INCA-FRc19x and Bacillus sp. INCA-FRc7 from muscovite (Fig. 4B). Only the strains Stenotrophomonas sp. INCA-FRr1 and Bacillus sp. INCA-FRc19x released К from К-feldspar and muscovite, respectively, under the four salinity concentrations tested.
Discussion
Potassium is part of the clay minerals that make up the mineral fraction of the soil; a soil rich in clay has a high content of К (Melgar & Castro, 2005). However, Red Ferralitic soils, considered clayey, are exceptions because the ferratilization process considerably reduces the total К content, as it does with the assimilable К content (Hernández Jiménez et al., 2010). As the К is depleted from the mineral interlayers of the soil, the release rate becomes progressively slower (Melgar & Castro, 2005), a process that increases with constant agricultural activity as is the case with the sampled soil. The use of К chemical fertilizers results in a strong adsorption of К to the minerals causing their contraction (potassium fixation) (Melgar & Castro, 2005; John-Louis et al., 2017) and making them inaccessible for the plants. This could explain the low amounts of assimilable К found in the sampled soil, especially in the soil furthest from the rhizosphere, which would allow the establishment of microbial populations with К solubilizing capacities. Atlas and Bartha (2005) state that the deficiency of a nutrient in the medium stimulates the microbial solubilization/fixation activity as a survival mechanism.
However, the content of assimilable К in the rhizosphere was slightly higher than in non-rhizospheric soil, which could indicate that at this level, the microbial activity of К solubilization has been stimulated, possibly influenced by radical exudates and plant-microorganism compatibility (Reinhold-Hurek et al., 2015). The microbial biodiversity of the rhizosphere is determined mainly by the composition of the radical exudates, which are specific to each plant species and decide the type and density of microbial populations associated with the plant (Verma et al., 2020) Additionally, the differences detected between the pH values in both soil zones indicate an acidification around the rhizosphere that would favor the solubilization of К minerals in this region (Verma et al., 2020).
Studies on OB from corn crops are scarce, and it is an interesting topic given the crop's high demand for К. Although OB are the most important members of the microorganisms involved in the К cycle (Sattar et al., 2019) , the few isolates obtained in this research highlight the exclusivity of this activity in the microbial world in general. The identified genera have previously been isolated from rhizospheric soil samples and roots of crops, such as soybean, rice, rape, apple, tea, Sudan grass, sorghum and wheat, and characterized as OB by different authors (Verma et al., 2016; Egamberdieva et al., 2017; Verma et al., 2017; Our, Rana, Yadav, et al., 2020; Rana et al., 2021). Considering that most of the OB that are known correspond to the phylum Proteobacteria (Our, Rana, Yadav, et al., 2020) , the genus Bacillus (phylum Firmicutes) is one of the most studied and best yielding (Baghel et al., 2020; Our, Rana, Yadav, et al., 2020; Sun et al., 2020). This supports the results obtained in this research where the strains INCA-FRc7 and INCA-FRc19x identified as Bacillus sp. showed the highest К solubilization index on solid Aleksandrov medium supplemented with К-feldspar. These strains also stood out in К release in liquid medium, showing the best yields under the different cultivation conditions, both with К-feldspar and with muscovite.
On the other hand, the К solubilizing capacity of the rest of the strains remained unchanged in the presence of both К mineral sources in solid medium, in accordance with the determinations of Nath et al. (2017), Nath et al. (2018), and Sun et al. (2020). The Lysinibacillus sp. INCA-FRc27 and Paenibacillus sp. INCA-FRc9 strains obtained the lowest К solubilization and К release indices in all the determinations; however, the values obtained did not correspond to those determined by Naureen et al. (2017) and Xiao et al. (2017) who characterize these genera as good К solubilizers.
The solubilization of К is mainly due to the action of organic acids (Ghadam Oani et al., 2019), both of microbial and plant origin (Verma et al., 2020). However, like all physiological processes, К solubilization is directly influenced by external factors such as temperature or pH that will determine the efficiency of the process (Verma et al., 2020). In this study, К release was most favored by a temperature of 30°C, a pH of 7.5 and the lowest saline concentration tested. These results are similar to those obtained by Sun et al. (2020) who obtained a maximum amount of К released from К-feldspar of 1.751 mg L-1, while the К release efficiency by the strains in this research was 13% higher with the same К source.
Verma et al. (2020) identified Bacillus, Paenibacillus and Pseudomonas strains with the ability to solubilize К in temperatures up to 60°C. The growth and К solubilization at the different temperatures determined in this study was lower in comparison with those results. The critical temperature was 40°C for all isolates in the presence of both К sources, and 37°C for Gram-negative strains in the presence of muscovite. Only the Stenotrophomonas sp. strain INCA-FRr1 managed to develop and perform in all four temperature ranges. Additionally, the acidic and neutral pH generally favored more К release, while alkalinity conditions only stimulated the release of К by the Arthrobacter sp. INCA-FRc17, Bacillus sp. INCA-FRc7, Pseudomonas sp. INCA-FRr15 and Stenotrophomonas sp. INCA-FRr1, coinciding with the results of Verma et al. (2020). Finally, salt stress affected К solubilization in both mineral sources. The level of halotolerance of the strains was limited to the lowest concentrations of NaCl, while at 12 g L-1 and 16 g L-1 the solubilization was very low or there was practically no microbial growth. However, Stenotrophomonas sp. INCA-FRr1 and Bacillus sp. INCA-FRc19x showed good resistance to these conditions, which corresponds to the results of Alexander et al. (2020) and Silambarasan et al. (2020), who characterized halotolerant strains of Stenotrophomonas with potential for promoting Lactuca sativa L. and Arachis hypogaea L. growth, respectively, and to Hamid et al. (2021) who obtained a К solubilizing Bacillus strain under salt stress conditions.
Yields of К release from both mineral sources showed important differences. Like the results of Verma et al. (2020) and Ghanbari and Safari Sinegani (2021), К release from muscovite was more efficient compared to К-feldspar in the same period of time; this result is interesting since muscovite is structurally more complex and less abundant in nature than feldspar minerals (Alling, 1923; Sattar et al., 2019). Many strains such as OB identified by other authors generally exhibit other mechanisms for promoting plant growth such as biological N fixation, solubilization of other nutrients, or phytostimulation (Vasanthi et al., 2018; Verma et al., 2020), so the role of these strains as agricultural inoculants would not be limited only to the contribution of К but would act in a wide range of ecological niches.
Conclusions
In the rhizoplane of corn plants established in leached Red Ferralitic soil, OB populations corresponding to the genera Bacillus, Arthrobacter, Paenibacillus, Lysinibacillus, Pseudomonas, and Stenotrophomonas coexist. The yields in the К release differed between strains and varied depending on the established abiotic conditions. The strains corresponding to the genera Lysinibacillus and Paenibacillus showed less efficiency as solubilizers, while strains Bacillus sp. INCA-FRc7 and Bacillus sp. INCA-FRc19x stood out for their performance in all determinations. These strains may be part of future biofertilizers for the corn crop, and this work constitutes the preliminary steps to achieve this.