Introduction
The global carnation market is projected to reach USD 3.266 billion by 2026, from USD 2.719 billion in 2020 (Global carnation sales market report, 2020). Fusariosis disease is a critical problem for carnation growers in Colombia as in most countries where carnations are produced. Fusarium oxysporum (FOX) f. sp. dianthi (Prill. and Delacr.) (Snyder & Hansen, 1940) has become the most significant pathogen in fusariosis disease in this crop (Arbeláez, 1987; Arbeláez, 1992; Borrero et al., 2009). Large losses can occur due to the easy spread of the fungi across the infected cuttings, the fast dissemination by diverse means, the persistence of the pathogen in the soil, and the high cost of control measures. All these causes confer special importance to this disease (Graces et al., 2001).
Lopez and Fergus described the carnation basal rot for the first time in 1965. Since then, growers have related the disease to F. roseum. More recently, the species F. roseum has been replaced by other species of Fusarium (Burgess et al., 1988). Arbeláez (1992) reported that both FOX and F. roseum could occur together in the same crop, with their masking symptoms making their identification difficult.
Fusarium verticillioides (Sacc.) (FVER) (Nirenberg, 1976) is an important phytopathogen in cereal crops that produces significant economic losses around the world. It attacks mainly corn, causing rotting in shoots, roots, and spikes (Bush et al., 2004). Filgueira et al. (2007) first reported FVER presence on carnation stems with basal rot symptoms. The objective of our study was to identify the etiologic agent that produces basal rot on carnation and to describe the development of the disease in the plants.
Materials and methods
Biological samples
More than 1,500 plants with basal rot symptoms were used to isolate the fungus. These plants were collected between the 2012 and 2018 from 12 different carnation farms, located at the Bogotá plateau in the municipalities of Cajicá, Madrid, Siberia, Chía, Suesca, Sopó, Facatativá, and Tocancipá. All of them were in the Cundinamarca department of Colombia. The commercial carnation varieties sampled were: 'Nelson®', 'Tasman®', 'Delphi®', and 'Pink Nelson®', with basal rot symptoms as previously reported by Arbeláez (1987) and Filgueira et al. (2007).
Fungi isolation
All the stem fragments, sampled at the crown level of infected plants, were surface sterilized with 5% sodium hypochlorite for 1 min and then with 70% ethanol for 1 min and rinsed several times with sterile water. Once surface disinfection was completed, the stem was sectioned into discs of 2 mm thickness, which were then grown on Potato Dextrose Agar (PDA) (MERK) and Czapek Dox (OXOID) and incubated at 25°C for 7 d. Once the pathogen colony was visible, it was transferred to liquid Czapek Dox Broth Sigma with agitation for a week at 25°C. Then, a single-spore culture was obtained from each isolation, three 1:10 serial dilutions of the spore suspension were made and 0.1 ml of the last dilution was taken and scattered on PDA. After incubation, a monosporic growth was selected and isolated on PDA. Finally, the fungi were conserved in glycerol 50% at 4°C. Colony characteristics such as color and appearance were observed in PDA cultures. The bright field in an Axioskop 2 Plus Carl Zeiss microscope was used to determine the presence, absence, and type of micro and macroconidia, the morphological characteristics of the mycelium, and possible formation of chlamydospores by the isolate. Asexual structures like macroconidia were measured using the Scion Image 4.0 free program.
Symptom characterization
On 53 carnation hybrid lines, the product of the crossing between varieties resistant and susceptible to FOX and one commercial variety 'Nelson®' hybrid lines and the commercial variety were established from seeds in vitro using MS basal media (Murashige & Skoog, 1962). One hundred microplants of each hybrid line and 'Nelson' variety were acclimated to outdoor conditions of the Bogotá plateau (maximum temperatures around 19 to 20°C [66 to 68°F] and minimum temperatures from 7 to 9°C [44.5 to 48°F]; the average annual relative humidity of 79%), with a vermiculite-like substrate. Four weeks later, plants of each hybrid line and commercial line were transplanted into pots. This evaluation used a quantitative measure of disease intensity with time (AUDPC) with analysis of variance by ANOVA in three repetitions that showed a normal distribution. The roots of each of the sixty plants were inoculated with 6 ml of a suspension of 1x106 spores/ ml (30 plants with FVER and 30 with FOX) using a syringe and injecting the fungus around the plant crown. Seven d after inoculation, the plants were re-inoculated with 200 µ1 of a suspension of 1x106 spores/ml by puncturing next to the stem base (Filgueira, 2011). As a control, 20 plants of each hybrid line and the commercial variety were inoculated in the same way with distillate water. Finally, 20 plants were used as negative controls without inoculation.
Weekly for 4 months, the plants were observed and a detailed record of the appearance of symptoms was performed. The criterion used for the external symptom registration was yellowing and the discoloration or loss of green color was measured as presence/absence. The wilting of the foliage, stem, and buds was measured according to the following scale: absence 0, wilting of the stem base and cracking 1, wilting that affects the stem and leaves that can be observed in % of plant 2, total wilting of the plant and death 3. Spots, such as stem discoloration, were measured as absence/presence. External necrosis, such as basal disintegration of the stem, was recorded as absence 0, moderate tissue damage 1, brown color in the stem base 2, and possible leaf necrosis, loss of turgor, stem decay, and dehydration 3. Finally, symptomatic plants were collected at random, and the pathogen was isolated in vitro to corroborate the entrance of the pathogen to the plant. The incidence was estimated as the number of plants that are diseased, relative to the total number collected (Campbell & Neher, 1994).
In vitro evaluation of the plant response to Fusarium presence
In vitro evaluation consisted of an essay of "dual" cultures, in which undifferentiated cells were obtained from microplants leaf explants of 53 carnation hybrid lines, cultivated on MS basal media supplemented with 1 mg L-1 of 2,4-Dichlorophenoxyacetic acid (2,4-D) (Filgueira, 2011). One gram of in vitro cells was placed in a Petri dish (diameter 90 mm) border, with the same media as the callus induction, and cultured for a week at 26°C. Next, 10 µ1 of spore solution of the pathogen (FVER or FOX) at a concentration of 1x106 spores/ml was inoculated on a sterile circle of filter paper on a Petri dish located opposite to plant cells and then left for two weeks. Possible inhibitory (resistant) or not-inhibitory (susceptible) responses by plant cells towards pathogen growth were recorded measuring the growth of the fungi in the direction of the plant cells in centimeters (Filgueira, 2011).
PCR identification
For this purpose, four of the characterized isolates of both FOX and FVER that were placed by rapid growth in the media and did not present other biological contaminants were used. Fungal DNA was extracted, and PCR amplification was performed following the method described by Cenis (1992), with the modification by Abd-Elsalam et al. (2003). The fungal conidia were inoculated in 10 ml of liquid Czapek Dox Broth (Sigma®) for 72 h at 25°C; later, the fungal mycelia were centrifuged at 15000 g for 5 min. Next, the samples were washed with 2 ml of Tris Buffer (TE), (10 mM Tris-HCl pH 8.0, 1 mM EDTA) and the mycelia were macerated in liquid nitrogen for 5 min. Three hundred µ1 of lysis buffer (200 mM Tris-HCl pH 8.5, 250 mM NaCl, 25 mM EDTA, 0.5% SDS) was added for 5 min, followed by the addition of sodium acetate 3 M (pH 5.2). The fungal samples were put at 20°C for 10 min and centrifuged at 15000 g for 5 min. The supernatant was transferred to a new tube and an equal volume of isopropanol was added and incubated at 4°C for 4 h. Finally, the suspension was centrifuged at 15000 g for 10 min. Ethanol 70% was added to the pellet to wash and then discarded. The DNA was suspended in 100 µ1 of TE.
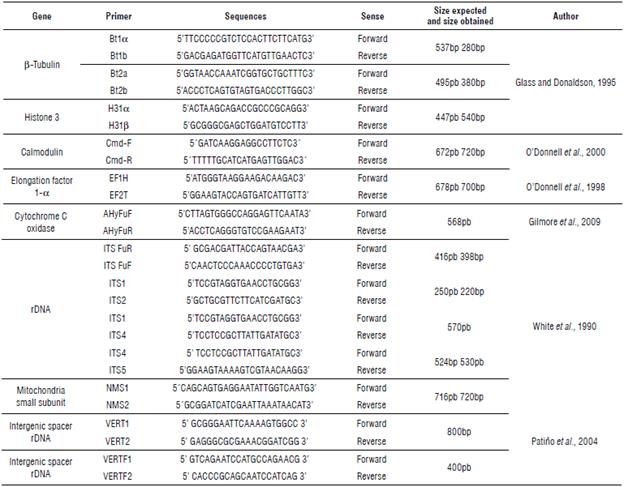
The primers used to identify the fungi are summarized in Table 1. The PCR mixture contained 5 to 10 ng of template DNA, 1 nM of each primer, 3 mM of MgCl2, 200 of dNTP, 2.5 U of Taq polymerase, and reaction buffer (50 mM KCl, 50 mM Tris-HCl; pH 8.3, 0.1 mg ml-1 BSA), in a final volume of 20 µ1. Thirty PCR cycles with the following temperature regime were performed at 95°C for 2 min (only in the first circle), at 94°C for 1 min, at the annealing temperature for 0.5 min, and at 72°C for 1 min. The PCR products were analyzed by electrophoresis on 1% agarose gel stained with 0.5 ml-1 of ethidium bromide. As an external group, DNA of Botrytis cinerea, obtained under the same conditions, was used. The outgroup is important as a point of comparison for the in-group and allows the phylogeny to be rooted. Each amplicon was sequenced twice (forward and reverse) using the Sanger et al. (1977) method by Macrogen® (Korea). The DNA consensus sequence was compared with the GenBank database. Sequences that presented similar values higher than 97% with the morphologic species, using the BLAST tool, were used to generate a consensus using CLC DNA Workbench® software. The consensus sequences were compared by multiple alignments with CLUSTAL OMEGA in the MEGA7 software (Molecular Evolutionary Genetics Analysis), which was used to build the phylogenetic tree using the Neighbor-Joining distance method.
TABLE 1 Forward and reverse primers employed. Thirteen amplicons belonging to eight different genes or genetic regions were used to identify the fungi taxonomically.
The DNA sequence data obtained in this study have been deposited in NCBI GenBank and the accession numbers are listed in https://www.ncbi.nlm.nih.gov/nuccore/?term=Fusarium%20filgueira.
Processing of data
All biological materials in this study were randomly selected and the data were handled with descriptive statistics for qualitative dichotomous characteristics by presence or absence (P/A) of symptoms of necrosis, spots, curl, sporulation, stem chlorosis, turgidity loss, rachis, rot, and decay. The data were processed in the MINITAB Release 17 program using a model of logistic regression for the P/A of symptoms. The variance between assay groups with the ANOVA program was evaluated, and then the data adjustment in the model was tested. All the data were studied in time (d). Using the previous method, the tendency of the symptoms in time was observed, and finally, the Tukey's range test was applied.
The information for sequences of the different loci used in this study was processed using the Multi-Locus Sequence Analysis (MLST) tool of the CBS-KNAW Fungal Biodiversity Centre's Fusarium MLST website (https://fusarium.mycobank.org/) and the corresponding Fusar-ium-ID site hosted at the Pennsylvania State University (http://isolate.fusariumdb.org). Dendrograms were generated by neighbour-joining and split decomposition with bootstrap analysis with 1,000 replications, using MEGAX.
Results
Symptoms in plants
In general, it is possible to distinguish fusariosis symptoms at the base of the plants in the crop field, e.g., FVER produces a dry rot that ascends towards the medial part of the stem, forming caverns-like cracks with or without jumps. These lesions did not compromise the vascular bundle (Fig. 1A-C). In advanced stages, it was possible to detect a white to reddish cottony mycelium inside the lesion (Fig. 1G). On the other hand, the infection with FOX in the basal portion (crown) of the plant was characterized by a lesion in the form of a narrow ring limited to the vascular system that did not compromise the medullary tissue in the first stage of the infection (Fig. 1D-E). In some cases, it was possible to detect both fungi infecting the same plant (Fig. 1F). Other external symptoms of basal stem rot caused by FVER are: partial or complete chlorosis, generalized or partial wilting green, stem turgor loss, cracking in the crown, presence of conidiation structures, necrosis, and finally plant death (Fig. 1H).

FIGURE 1 Symptoms of fusariosis in carnation plants. A-C) transversal and longitudinal cuts in the crown of carnation plants with the symptom of basal rot caused by FVER (C initial stage of invasion and B final stage); D-E) Transversal and longitudinal cuts in plants with symptoms of vascular wilt caused by FOX; F) Plant infected by both parasites with the symptom of basal rot (black arrow) and vascular wilt (red arrow); G-H) Carnation plants that present the final phase of the disease caused by FVER with cracking in the crown, the presence of fungal structures, and necrosis.
The analysis of the fusariosis disease under field conditions showed that the Pink Nelson variety presented a higher FVER incidence of 7.1°% (SD=1.2), followed by the Delphi variety with 3.7% (SD=0.6) and the Nelson variety with 3% (SD=0.5) incidence. In the case of FVER, the maximum incidence observed was 10% (SD=1.6) as opposed to FOX, with a maximum incidence value of 68% (SD=5.7) in the farms.
Fungal isolation and preliminary characterization
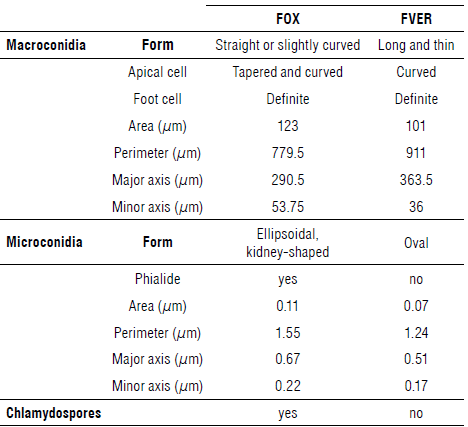
The evaluation of 1,500 samples of monoconidial culture showed that, in 68.3% of the samples presenting symptoms of fusariosis, FOX was present, while in 8.2% of the samples, FVER was present, values close to that of the infected plants that were observed in the field. Ten percent of the samples presented both species simultaneously; 11.5% of the plants presented other Fusarium species (F. graminearum, F. foetens, F. culmorum, F. avenaceum, and Fusarium sp.), and, in 2% of the cases, it was not possible to identify which of the parasites was present. Microscopically it was possible to identify septate mycelium in FVER isolates and production of long and slim macroconidia with thin walls, bent apical cells, and basal cells in the form of foot, with three to five septa. The morphological characterization of vegetative and reproductive structures of FOX and FVER is presented in Table 2. Macroconidia of FVER are shown in Figure 2A-B. Figure 2D shows observed simple branched FVER monophialides and abundant ovoid microconidia. The microconidia were only obtained on CLA (carnation leaf agar) media after 45 d of cultivation (Fig. 2B-C) and occurred separated from each other or in short and long chains (Fig. 2C). This last characteristic is important for distinguishing FVER from FOX. In this study, dark yellow sporodochia were observed in FVER in vitro media with more than 45 d of cultivation.
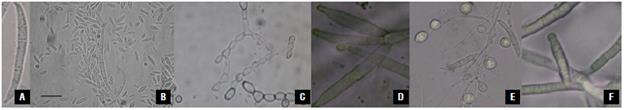
FIGURE 2 Isolation of FVER and FOX in nutrient media and microscopy recognition. A) Macroconidia of the FVER; B) FVER Macroconidia and microconidia. Bar is one jm; C) Production of FVER microconidia in a chain, 100X; D) FVER branched monophialide, 180X; E) Macroconidia, microconidia, and chlamydospores of FOX. Monophialides of FOX. A, C, D, and F, 1100X. B and E, 750X.
Tests of pathogenicity
In vitro interaction
Through the different tests, we study the processes of recognition and invasion of carnation plants by FVER. The first test was an in vitro assay, where the pathogen and undifferentiated cells of the plant can mutually recognize each other. In this case, it was possible to observe that the mycelia of the fungi grew mainly in the direction in which the plant cells were present (Fig. 3A, Fig. 4A). In the opposite case, where the cells present a resistant reaction to the presence of fungi, the fungi mycelia grew more slowly in the direction of the plant cells, as a type of growth inhibition reaction (Fig. 3B, Fig. 4A.). This evidences a host-parasite interaction between carnation cells and FVER. This behaviour was also observed in an in vitro assay with FOX (Fig. 3C-D., Fig. 4B).
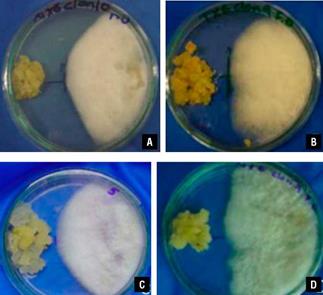
FIGURE 3 "Dual" culture assay in vitro. A) In the interaction between susceptible carnation cells and FVER, the fungus grew towards the carnation cells; B) Interaction between carnation-resistant cells and FVER indicating that the fungi stopped its growth by the presence of the resistant plant cells. An assay using FOX with carnation-susceptible cells C) and resistant cells D).
To determine if the daily growth of the funguses in presence of resistant and susceptible carnation cells differs significantly with the "dual" culture in the Petri dish, variance analysis was done by d using ANOVA program with a P<0.05. The results show significant statistical differences between the fungi growth in the presence of resistant cells and susceptible cells (Fig. 4A-B).
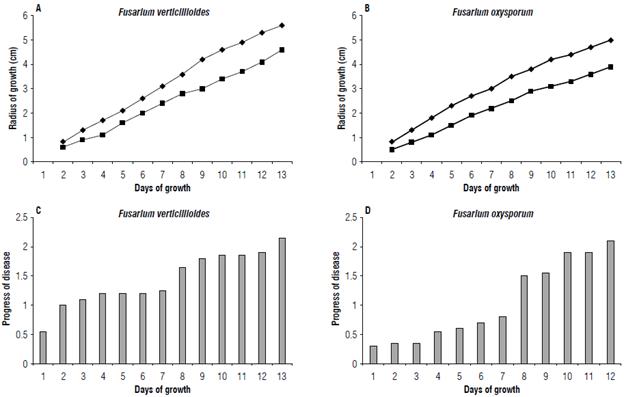
FIGURE 4 Fungi growth in vitro (A and B). Disease progress in the ex vitro test (C and D); A) FVER growth and B) FOX growth. The fungus growth is shown on in vitro assay ("dual" culture), in the carnation resistant cells interaction (■) with the susceptible cells (♦); C) Plants inoculated with FVER and D) plants inoculated with FOX. The plant wilting progress is recorded in days in the assay of plant inoculation in the greenhouse.
Ex vitro interactions in the greenhouse
In the inoculation test of susceptible plants inoculated with FVER in germination trays, the first symptom of dry stem bark at the plant base was observed 15 d after the first inoculation. This symptom became most prominent and ascended between 10 and 15 cm from the stem base by week 10 to 13. The Tukey's test presented a value of 3.45 (a=0.05, K=3, df = 5) near week 7. It was possible to observe wilt with foliar yellowing on the leaves located near the plant base of the susceptible varieties. In this case, the Tukey's range test showed a value of 2.95 for this symptom by week 5. Additionally, internally in the plant base, a continuous or discontinuous pattern of rots at the same zone on the stem medulla was found, as shown in Figures 1A and C., which was near d 30 after the first inoculation. This symptom reached a value of 3.4 in the Tukey's test near week 10. The rot spread on the stem base from the modular zone toward the vascular bundles and other stem tissues (Fig. 1B). The rots inside the stems sometimes appeared with red stains located in the modular zone, and wilting was evident from week 3. The Tukey's test showed a value of 3.95 for this symptom after week 7.
Likewise, the spot symptom in the base stem indicated pathogen colonization, which was evident between weeks 3 and 4 after the inoculation. The Tukey's test showed a value of 3.85 to 3.95 after week 10. The tissue necrosis symptoms were present posterior to the spot apparition, near week 4 (Fig. 1G). Plant behavior was similar in all the control groups. A comparative study with FOX (data not presented here) indicated a significant difference in the infection progress of the disease (Fig. 4C-D), such as the plant wilting progress over time. These data determine that FVER is more aggressive in the infection and colonization of the plant stem than FOX.
As mentioned above, we selected symptomatic plants randomly for each inoculation treatment and the re-isolation in vitro of the pathogen to confirm the presence or absence of the isolates, fulfilling Koch's postulates. Twenty plants of the susceptible commercial variety Nelson were re-inoculated with conidia of the pathogen obtained from infected plants from the first inoculation experiment. All these plants presented severe symptoms of basal rot with foliar yellowing, vascular chlorosis, medullar rot, and stem cupping. Fragments of this infected tissue were used to isolate the parasite and determine the presence of the fungus that was used to inoculate the initial plants like FVER.
Molecular identification
An adequate quantity of genetic regions was employed to appropriately identify the two species because of the problems in using morphological characters in the identification of FOX and FVER. First, we used the ITS region for the identification of the genus to resolve the limitation that this genetic region presents for use as barcoding. Next, we used a group of primers to determine with the greater accuracy of the fungal species, especially when the study implicates phylogenetic analysis (Tab. 1). In this sense, the use of MLST is the best option to identify and obtain phylogenetic relationships between species of difficult identification (Matsumura, 2013).
Ten samples of each of the morphological species FVER and FOX were selected. To prevent slants or false positives, a preliminary molecular characterization by PCR using ITSs and EFs 1- α (Tab. 1) was performed to determine if the isolates previously classified as FOX or FVER belong to the Fusarium genus. Figure 5A-E shows the amplicons of the primers ITSFuF/ITSFuR, ITS1/ITS2, ITS1/ITS4, ITS4/ITS5, and EF-1H/EF-2T. The accession number (ID) to view the nucleotide sequence in the GenBank of NCBI of these amplicons is listed in Table 3. In most cases, such as amplicons ITS1/ITS2 and Elongation Factor 1-α, the identity of these sequences with different sequences of F. verticillioides (e.g., ID XR_001989346.1) in GenBank was 100% and the e-value 0.0.
TABLE 3 GenBank (NCBI) IDs and comparisons with related sequences. The DNA sequences of FVER and FOX obtained in this study were compared using the BLAST tool, and the results show the identity percentage and the E value of the most related sequences.
| Locus | Gene | FVER* | FOX* | Related specie | Percentage Identity | E value |
|---|---|---|---|---|---|---|
| ß-Tubulin | Bt1 | KF467337.1 | KF467331 | F. vertcillioides | 100 | 0.0 |
| ß-Tubulin | Bt2 | KF467337 | JF773354 | F. vertcillioides | 97 | 0.0 |
| Histone 3 | H3-1 | KF467389 | KF467383 | F. vertcillioides | 100 | 0.0 |
| Calmodulin | Cmd | KF467350 | KF467344 | G. moniliformis | 99 | 0.0 |
| Elongation factor 1-a | EF | KF467376 | KF467370 | F. verticillioides | 100 | 0.0 |
| Cytochrome C oxidase | AHyFu | KF467363 | KF467357 | G. moniliformis | 99 | 0.0 |
| rDNA | ITS Fu | KF467402 | KF467396 | F. verticillioides | 100 | 0.0 |
| rDNA | ITS1-2 | KF467415 | KF467409 | F. verticillioides | 100 | 0.0 |
| rDNA | ITS1-4 | KF467428 | KF467422 | F. verticillioides | 100 | 0.0 |
| rDNA | ITS4-5 | KF467441 | KF467435 | F. verticillioides | 100 | 0.0 |
| Mitochondria small subunit | NMS | KF467454 | KF467448 | F. verticillioides | 100 | 0.0 |
| Intergenic spacer rDNA | VERT | submitting | submitting | G. moniliformis | 97 | 0.0 |
| Intergenic spacer rDNA | VERTF | submitting | submitting | G. moniliformis | 95 | 1 e-121 |
Other genes used in this study permitted a more adjusted taxonomic classification. The accession numbers of Gen-Bank (NCBI) of the sequences obtained in this study with the different genes used are listed in Table 3. In the same way, Table 3 lists the identity percentages and the E values produced by the comparison between the sequences obtained in this study with the sequences of the closest taxonomic species, using the BLAST tool of NCBI.
Using the sequences obtained with the different amplicons, a MultiLocus Sequence-Typing (MLST) schema was built. The MLST schema used an agglomerative clustering of a complete linkage of eight different genes of FVER (EF1, Cmd, Bt1, VERT, VERTF, ITS1-2, ITS Fu, and NMS). The eight genes of FVERT were compared with thirty-one conserved housekeeping genes to derive a combination of alleles known as a sequence type (ST), using the Fusarium-ID site hosted at the Pennsylvania State University (Fig. 5). To obtain the MLST, we used the most representative FVER sequences, which obtained the highest percentage of identity and the lowest E value in the sequence alignment using the BLAST tool, as seen in Table 3. The MLST schema shows that the isolate that previously identified F. verticillioides is clustered with similar types like Fusarium verticillioides (Fusarium fujikuroi species complex), excluding Fusarium oxysporum (Fusarium oxysporum species complex) that are grouped in a different phylogenetic group (Fig. 5).
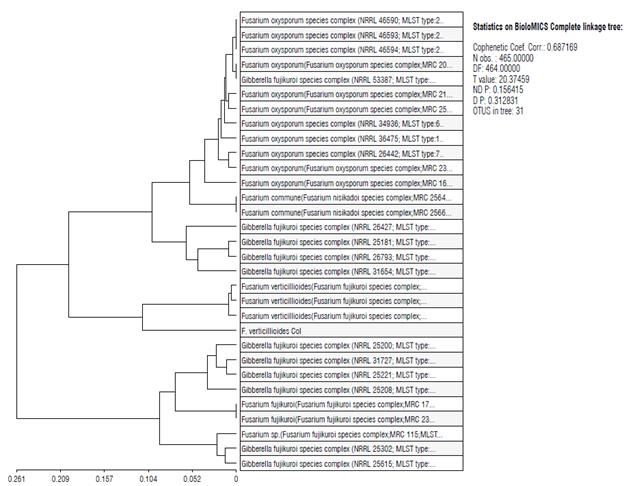
FIGURE 5 MLST scheme. FVER (F. verticillioides col) phylogeny inferred from an EF1, Cmd, Bt1, VERT, VERTF, ITS1-2, ITS Fu, and NMS dataset genes, in which six species complexes were resolved. The approximate number of phylogenetically distinct species within each species complex is based on an agglomerative clustering of complete linkage analysis.
Discussion
Symptomatic and morphological recognition
This study showed that FVER is a pathogen of the carnation, which can lead to a confused diagnosis with carnation plants infected with FOX. Here, we demonstrated that FVER attacks carnation plants, producing some symptoms like those of carnation plants attacked by other Fusarium species. The morphological description of the species FVER agreed with those of Leslie and Summerell (2006) and other authors (Deepa et al., 2018), with the presence of typical macroconidia, microconidia in a chain, and absence of chlamydospores.
In vitro tests showed resistance to FVER from cells of different hybrids and commercial varieties. This demonstrates that the plant can recognize FVER and elaborate a response to it. The pathogenicity test, the in vitro "dual" test is based on the chemical response, in which recognition of the pathogen activates the metabolism of defense in the plant cell wall (Bigeard et al., 2015). In this assay, the undifferentiated cells of the hybrid lines, which have some type of resistance towards the pathogen, induced smaller displacements on the fungus on the nutrient media, supporting the hypothesis that antifungal molecules are produced by callus into the media (Buiatti et al., 1987). This type of response has been already reported, characterizing lines of cells of tomato and evaluating them in vitro responses against FOX. In this case, a correlation between phytoalexin production and inhibition of fungal growth was reported (Storti et al., 1989). In our case, in some "dual" tests, changes were presented in the form of the fungal growth front line. This clearly shows a response by the callus, which does not allow the fungi to continue its normal advance. Filgueira and Guardiola (1999) also reported this change in the growth direction of the fungus as a response to the resistance of the carnation callus to the pathogen presence. Several reports have shown similar results in their studies, such as the measured severity and disease incidence. They show correlations between the in vitro assays and those observed in the field (González et al., 2004).
In the carnation, FVER produces basal rot, which is characterized as dry rot in the base of the stem, as previously observed in maize by Leyva-Madrigal et al. (2015). Sometimes the rot can be presented in phases as Filgueira reported in 2007 in a study of the carnation, and when the infection is advanced, cottony mycelium can be seen in the cave produced by the medullar rot. Also, posterior expansion of the infection is possible toward the vascular bundles and other stem tissues, producing stem rot disease. Externally, the stem in the base presented chlorosis, cortical necrosis, dry rot, and yellowing foliage.
Symptoms of the disease caused by FVER expand in the plant from bottom to top, similar to wilting caused by FOX. These two pathologies can be differentiated doing cross-section cuts to determine if necrosis is present in the medullary zone of the vessel bundles. In addition, it should be kept in mind that FVER does not produce an asymmetric yellowish color on the leaves. On the contrary, the leaves lose hydration, and the wilting shows a strong pink color in the stem. The FVER sporulation on the outer side of the stem is remarkably similar and consistent with rot caused by FVER in corn, where the rot is accompanied by sporulation (Rossi et al., 2008).
In carnation plants, rot in the medullar zone can also be observed when attacked by species of Fusarium other than FOX, such as FVER, as reported by Filgueira et al. (2007). This suggests that the presence or proportion of these pathogenic species depends on the individual incidence of each pathogen. The greatest incidence of double infections was present with FOX and FVER together. These species colonizing the same plant evidenced low or nonexistent ecological competition among the species. This result evidences a possible synergy between some of the species and stem colonization. Additionally, the presence of Fusarium in symptomatic plants was found in some carnation varieties.
Molecular identification
In all the cases analyzed using ITS primer, all the corresponding to the Fusarium genus were demonstrated in other cases by Glass and Donaldson (1995) and Tan and Niessen (2003). Furthermore, the amplicon obtained with the primers ITS 1/2 consistently showed that the species most related with the analyzed sequences was FVER. In general, the obtained size of the amplicons of this study did not differ significantly from the expected size, except for the case of ß-tubulin which showed an appreciable difference (Tab. 1); the difference was not a problem when using the sequence as a tool to identify the related species in the GenBank. Most of the amplicons obtained with the different primers of the genes used in this study were identified as the species most related to FVER. This is the case of the ß-tubulin, the Bt1a/Bt1b and Bt2a/Bt2b amplicons, the cytochrome C amplicon, histone-3, calmodulin, and mitochondrion small subunit amplicon, which presented identities above 97% and evalues in all cases near zero.
The product obtained by the primers VERT1/VERT2 and VERTF2/VERTF specific to identify FVER produced bands of the expected sizes of 800 and 400 bp, respectively, as reported by Patino et al. (2004) in the isolates of FVER from the symptomatic flowers of farms. This is indicative of the species with Fumonisin gene presence. In addition, Fumonisin is a mycotoxin produced by some Fusarium species like FVER (Scott, 2012; Rosa Junior et al., 2019).
On the other hand, the phylogeny analysis with the MLST schema using the genes of FVER EF1, Cmd, Bt1, VERT, VERTF, ITS1-2, ITS Fu, and NMS produced a tree with a clear aggregation of FOX isolates in a separate group of FVER species and with an important genetic distance (Fig. 5). All these results evidence that 1) FVER is present in carnation growing fields. This is notable because FVER is an important parasite of different crops of economic importance like cereals; 2) FVER has a type host-pathogen relationship with the carnation that had not been studied until now and produces notable symptoms in the plant leading to the die; 3) the disease known as F. roseum by carnation growers is a basal rot caused by FVER; 4) FVER and FOX can act synergistically to produce an infection of the plant that presents symptoms of both disease, vascular wilt, and basal rot; and 5) FVER is producing an emergent disease that could be related to climate change and must be acted upon.