Introduction
Sonchus oleraceus L. belongs to the Asteraceae family and has an annual life cycle, growing up to 1.5 m in height (Elkamali et al., 2011). The plant is characterized by a whitish latex and has alternate simple leaves and inflorescences with yellow terminal capitulum (Fuentes et al., 2011; Gámez et al., 2018). This species has different common names in Spanish, among them is cerraja, lechosa or lechuguilla. In English, it is known as dandelion sow thistle and hare's thistle.
S. oleraceus is widely distributed throughout the world and, in Colombia, it is present in the highlands of almost the entire territory (Fuentes et al., 2011) and is considered invasive (Peerzada et al., 2019). As a weed, it has been reported in corn, grasslands, oats, vegetables, asparagus, garlic, feijoa, pea, quinoa, apple, leek (Gámez et al., 2018), among others.
In peach orchard under tropical conditions, it was reported as the species in fifth place with the highest importance value index (Moreno-Preciado & Balaguera-López, 2021). S. oleraceus is of great interest to cause a detrimental impact on agriculture through crop competition and allelopathic interference (Chauhan et al., 2006; Elkhayat, 2009). It is a limiting species for crops because it has a great competitive capacity and produces a high number of most viable seeds (Peerzada et al., 2019). It is also considered the host of different pests and diseases, such as aphids, fungi, nematodes, besides other organisms that affect crops of economic interest (Gámez et al., 2018). Genetic diversity, efficient seed dispersal, resistance to some herbicides (groups M and B) are some of the factors that contribute to the successful distribution of S. oleraceus in the agroecosystems. In Australia, it is currently considered the second most important broadleaf weed (Peerzada et al., 2019).
Herbicide use and herbicide resistance hinder weed management (Guglielmini et al., 2007; Vencill et al., 2012) and cause a high negative impact on crop yields (Menalled, 2010), environment, and human health (Böcker et al., 2019). Therefore, new management alternatives must be found, within which biological management can be included (Peerzada et al., 2019) based on the use of allelochemicals (Jabran, 2017). Allelopathy is a tool that allows for the sustainable management of weeds (Arafat & Ali, 2015), allowing the maintenance of a balance in the ecosystem by reducing the use of chemical herbicides (Nawaz et al., 2014; Kaab et al., 2020). Allelochemicals (including phenolic compounds, terpenoids, and alkaloids) can be formulated into bioherbicides, but many aspects that should be studied are still unknown, such as an understanding of their mode of action (Jabran, 2017).
The champa (in Spanish) or perfume guava (in English) (Campomanesia lineatifolia R. & P.) is a fruit tree of the Myrtaceae family. Its fruits are edible berries with a pleasant taste (Balaguera-López et al., 2012; Porras et al., 2020; González et al., 2021), containing 6 to 8 seeds that represent an important percentage of the fruit volume (Balaguera-López et al., 2012). The seeds appear to have a high and diverse content of secondary metabolites. Bonilla et al. (2005) found that the seed of C. lineatifolia has ß-tricetone-like components, which are characterized by the presence of several methyl groups, a flavonoid ring or chalcone, making it a relatively rare class of secondary metabolites. These compounds are known to have antimicrobial activity (Bonilla et al., 2005; Osorio et al., 2006). Munoz et al. (2015) report in this species the existence mainly of diphenols and polyphenols, Madalosso et al. (2012) mention the presence of flavonoids (catechin and quercitrin) and tannins, while Raphaelli et al. (2021) report that the C. xanthocarpa seeds contain phenolic compounds with different biological activities. The same authors also indicated that the species of the Myrtaceae family are recognized for having high levels of the bioactive compounds, mainly phenolics and carotenoids.
Some plants have biologically active compounds, whose crude, methanolic, and other allelopathic extracts inhibit the germination of weed seeds and affect plant growth causing necrosis or chlorosis (Kaab et al., 2020). Several studies with allelopathic extracts have been carried out on Myrtaceae. Verdeguer et al. (2009) mentioned that the essential oil of Eucalyptus camaldulensis inhibits the germination and growth of Amaranthus hybridus L. and Portulaca oleracea L. Puig et al. (2018) observed that the aqueous extract of Eucalyptus globulus presents phytotoxic effects in Amaranthus retroflexus L. and Echinochloa crusgalli L. Recently, an allelopathic effect of C. lineatifolia seed extract was found on the germination and physiology of Taraxacum officinale L. (Cabeza-Cepeda et al., 2021).
Therefore, the objective of this study was to evaluate the allelopathic activity of C. lineatifolia seed extract on the germination, seedling growth, and injury of S. oleraceus L. under laboratory conditions.
Materials and methods
The experiment was conducted in Tunja (Boyacá, Colombia), under laboratory conditions, with a mean temperature of 19°C and relative air humidity of 60.8%. S. oleraceus L. seeds were obtained from farms in the municipalities of Tunja and Paipa (Boyacá). C. lineatifolia seeds were collected from mature fruits in the Miraflores municipality (Boyacá) in "La playa" farm located at 1432 m a.s.l., 5°11'49" N and 73°08'47'' W with a mean temperature of 24°C (González et al., 2021).
The ethanolic extracts of the C. lineatifolia seeds were obtained using the following procedure. Initially, the seeds were dried at room temperature (18°C) for 24 h, then this material was grounded, and weighed to obtain the extract. A 400 g sample of the grounded material was immersed in 1 L of ethanol (96%), the mixture was shaken and then left to stand in a glass bottle and covered with aluminum foil for a period of 48 h. The bottle was shaken regularly to homogenize the sample. Subsequently, the liquid was separated from the solid part through a filtering process (Whatman 1 filter paper), then it was subjected to a distillation process with a rotary evaporator to obtain the extract. From the concentrated extract, the different evaluated concentrations were prepared (% w/v) using distilled water as the final solvent. The study was carried out in two phases.
In the first phase, which corresponded to application on pre-emergent plants, germination was evaluated with two experiments. The first experiment consisted of the application of C. lineatifolia extract every third day. In the second experiment, the extract was only added at the time of sowing. In both experiments, a completely randomized design was used with four treatments corresponding to concentrations of C. lineatifolia seed extract (0%, 3%, 6%, and 9%), concentrations evaluated previously by Cabeza-Cepeda et al. (2021). Each treatment had four replicates and 16 experimental units; each unit consisted of a Petri dish with 100 S. oleraceus seeds.
In the second phase or the post-emergent application, the extracts were applied over the leaves of the plants. A completely randomized design with the same four concentrations of C. lineatifolia seed extract was used. Each of the 16 experimental units was composed of a pot with five S. oleraceus plants.
The seeds were sown in Petri dishes, on the base of which there was absorbent paper. Periodically the seeds were hydrated with the allelopathic extracts, except for the control that was hydrated with distilled water. In the second experiment, periodic hydration was done with distilled water. The Petri dishes were left at room temperature (19°C) and relative humidity (60.8%) with a 12 h photoperiod. Germination readings were carried out every third day from the moment the first seed germinated. It was considered as germination criterion that the radicle measured a minimum of 2 mm. From these data and with the formulas used by Carranza et al. (2016) and proposed by Ranal and Santana (2006) the percentage of germination and the mean germination speed (germinated seeds/d) were calculated. At the end of the experiment, the height (cm) and the length of the longest root (cm) of each seedling were determined by measurement with a Vernier caliper.
For the second phase, ten seeds of S. oleraceus were sown in pots with a volume of 500 ml, and peat moss was used as substrate. When the seeds germinated, 5 seedlings of similar size were chosen, and the rest were eliminated. Irrigation was carried out periodically, and fertilizer was applied weekly to avoid nutritional deficiencies. The fertilizer mixture was prepared by taking 20 ml of commercial fertilizer 12-4-8 (Follaje®, Davalia Fertilizantes, Colombia) and dissolving it in 2 L of water. Of this solution, 40 ml per bottle was applied. When the plants had between 4 and 5 true leaves, the extracts were applied uniformly to the leaves of the entire foliage using a 10 ml atomizer. It was necessary to add an adjuvant (Cosmoflux® 411F, Cosmoagro, Colombia) to the extracts to achieve uniform coverage on the leaves.
At 2, 5, and 8 d after the application of the extracts, the following variables were determined: incidence of damage (%), defined as the number of plants with damage by the herbicide/total number of plants x 100. The severity of the damage (%) was determined with the scale used by Chaves Neto et al. (2020), where 0-20% indicates no injury or effect, 20-40% slight injury and/ or growth reduction with rapid recovery, 40-60% injury and/or growth reduction with slow or definitive recovery, 60-80% severe injury and/or reduction of non-recoverable growth and/or reduction of the stand, and 80-100% destruction or just a few live plants.
Statistical analysis
An analysis of variance was carried out with the data obtained to determine statistical differences. Where differences were observed, the Tukey's test (P<0.05) was used. Variables that did not meet the assumptions of normality of the residuals (Shapiro-Wilk test) and homogeneity of variances (Levene test) were subjected to arcsineVx + 1 transformation and Vx transformation. The analyses were carried out with the statistical program SPSS IBM v. 19.
Results and discussion
Phase 1. Pre-emergence treatments
Germination percentage: there were significant differences between the concentrations of the extract and the control (without the extract) in the two experiments. Germination of S. oleraceus seeds with C. lineatifolia extract was found to be inhibited (Fig. 1). In agreement with these results, this extract also inhibited germination in T. officinale (Cabeza-Cepeda et al., 2021).
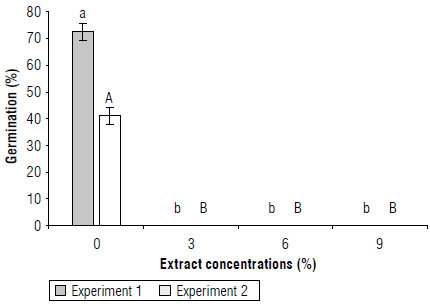
FIGURE 1 Bioherbicidal effect of the allelopathic extract of C. lineatifolia seeds on the germination percentage of Sonchus oleraceus seeds. Experiment 1: Continuous application of the extracts. Experiment 2: application of the extracts only at the sowing of the seeds. Means followed by different letters in each experiment indicate significant differences according to the Tukey test (P<0.05). Vertical bars for each mean indicate the standard error (n = 4).
The total inhibition of germination obtained with C. lineatifolia extracts may be due to the presence of secondary metabolites that directly affect the cells and cause the death of the seeds. C. lineatifolia seeds contain several compounds, such as catechins, phenolic acids, chlorogenic acid, flavonoids and anthocyanins, mainly (data not shown). This composition is according with that reported in other studies previously. According to Catunda et al. (2002), germination can be inhibited by the secondary metabolites (phenols, terpenoids, saponins, etc.) by affecting several fundamental processes such as respiration, cell division, synthesis of gibberellins, permeability of the seed coat, and other effects. Carvalho et al. (2019) reported that allelochemicals affected germination by inhibition of cell cycle in meristematic cells. To clarify this situation, it is recommended to perform a seed viability test with triphenyl tetrazolium chloride on seeds that did not germinate; if the test is positive, germination would be inhibited but the embryo is still alive; if the test is negative, this would indicate that the extract of C. lineatifolia caused the death of the seeds.
Imatomi (2010) evaluated the allelopathic activity of aqueous extracts of leaves of 15 species belonging to the Myrtaceae family on the germination and growth of Lactuca sativa, Solanum lycopersicum, and Allium сера, and found that the species Myrcia multiflora, Myrcia splendens and Eugenia punicifolia exhibited the highest inhibition of seed germination in bioassays, followed by the species Myrcia bella, Psidium laruotteanum, Campomanesia pubescens, Psidium cenereum, Eugenia myrcianthes, Myrcia lingua, and Psidium rufum. This supports the results obtained in this study where C. lineatifolia of Myrtaceae has an allelopathic effect that inhibits germination. However, knowing with certainty how the applied extract works is very difficult since the extract can have a significant number of different secondary metabolites that can have allelopathic action. According to Jabran (2017), the chemical inhibitors contained in plant extracts produced by allelopathic agents correspond to secondary metabolites and belong to various classes of compounds such as phenols, aldehydes, glucosides, terpenes, and organic cyanogenic compounds.
Gil et al. (2010) reported that the application of the extracts of Swinglea glutinosa and Lantana camara inhibited the germination of Senna obtusifolia, Amaranthus dubius, Rumex crispus, Brassica rapa, and Poligonum segetum. In the case of Swinglea, the extracts inhibit germination with the concentration 0.5% and Lantana inhibits germination with an extract of 2.0% (Gil et al., 2010). Seabra et al. (2017) evaluated allelopathic concentrations of Canavalia ensiformis on the seed germination of Carthamus tinctorius, where the inhibition was obtained with an extract concentration of 75% indicating that the extract of C. lineatifolia is very efficient because it needed only 3% to inhibit germination.
For mean germination speed (MGS), in the two experiments, statistical differences were found between C. lineatifolia extracts. Any concentration inhibited the germination of S. oleraceus; and, therefore, there was no germination speed. With the control (0 % of the extract) the seeds had a MGS of 5 germinated seeds/d for experiment 1 and 3 germinated seeds/d for experiment 2 (Fig. 2).
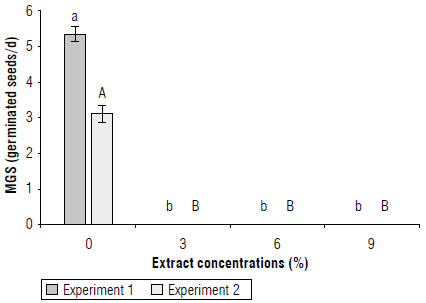
FIGURE 2 Bioherbicidal effect of the allelopathic extract of C. lineatifolia seeds on the mean germination speed (MGS) of Sonchus oleraceus seeds. Experiment 1: Continuous application of the extracts. Experiment 2: application of the extracts only at the sowing of the seeds. Averages followed by different letters in each experiment indicate significant differences according to the Tukey test (P<0.05). Vertical bars in each mean indicate the standard error (n=4).
This shows that the extract of C. lineatifolia caused phytotoxicity in the seeds of S. oleareaceus, which (as explained above) could be due to compounds derived from secondary metabolism such as phenolic compounds. That affect the growth and development of plants (Jabran, 2017), which, in this case, are the seeds.
Seabra et al. (2017) indicate that the allelopathic effects of plants can cause inhibition of the percentage and the germination speed and can reduce initial growth. This agrees with this study since the evaluated extract negatively affected germination. For their part, Gindri et al. (2020) found that the aqueous extract of Lantana camara causes inhibition of the germination speed index in Bidens pilosa seeds.
Length of roots and stems: since the extract of C. lineatifolia inhibited the germination of S. oleraceus, growth in seedlings that could only be measured in the control treatment, significant differences were observed concerning the other concentrations of the extract. On average, the seedlings had 2 leaves (Fig. ЗА), root length was 1.98 cm and stem length were 0.18 cm (Fig. 3B). The number of leaves was 2 because only the cotyledonal leaves were present at the time of the experiment. The stem was very short at that time, the longest organ was the root. In addition, the stem had a reddish-green color and the leaves were completely green. The allelopathic effect of the extract on seedling growth cannot be evaluated because the effect was 100% inhibition in the germination process. In another study, the phenolic compounds were responsible for inhibiting the growth of the Echinochloa crus-galli root (Chon & Kim, 2004), while Gindri et al. (2020) indicate that the triterpenes lantadene A and В from the aqueous extract of Lantana camara causes growth inhibition in epicotyl and root of Bidens pilosa.
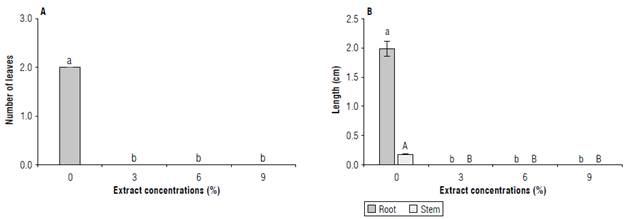
FIGURE 3 Bioherbicidal effect of the allelopathic extract of C. lineatifolia seeds on the number of leaves (A) and the stem and root length (B) of Sonchus oleraceus seedlings. Means followed by different letters indicate significant differences according to the Tukey test (P<0.05). Vertical bars for each mean indicate the standard error (n=4).
Gil et al. (2012) found that with Swinglea extracts there is complete root inhibition in the five weeds that they evaluated, which is consistent with the negative effect on germination. If there is no germination, there is no radicle protrusion and growth, as occurred in the present study.
Phase 2. Post-emergence treatments
Incidence (%): from 2 d on it was evident that all the plants showed symptoms of damage from the extract of C. lineatifolia, which is why 100% of incidence was evident. At 5 and 8 d, 100% incidence of damage continued to be observed (Fig. 4). The same values were found in T. officinale with applications of C. lineatifolia extract (Cabeza-Cepeda et al, 2021). As seen in Figure 5, the symptoms were localized yellowing and necrotic spots, showing that C. lineatifolia has potential as a bioherbicide. In this regard, Khan and Khan (2015) found that the extracts of several plants decrease the density of weeds to one-third about control in a wheat crop with the application of Ammi visnaga and Convolvulus arvensis.
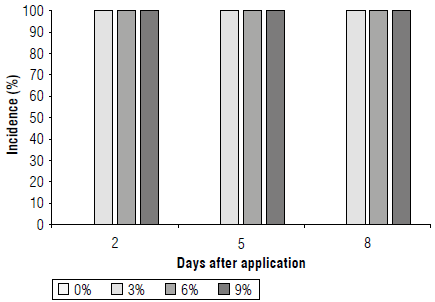
FIGURE 4 Bioherbicidal effect of the allelopathic extract of C. lineatifolia seeds on the incidence of damage in Sonchus oleraceus plants.

FIGURE 5 Symptoms of damage in S. oleraceus leaves caused by application of C. lineatifolia ethanolic extract.
For severity (%), significant differences were found throughout the study and the damage was greater as the concentration of the extract increased. At 2 d after application (first reading) the damage with the extracts was over 40.0 ± 0.4%; at 5 d (second reading) with 9% of the extract, 63.0 ± 2.0% damage was reached, and at 8 d (third reading) the damage with this treatment was 74.5 ± 1.7%. The other extracts produced lower effects but showed representative damage (Fig. 6). Although the plants did not die and some even tended to start flowering, perhaps due to the stress produced by the extract, this shows that C. lineatifolia exerts an allelopathic effect on S. oleraceus, apparently, due to the presence of phenolic compounds, therefore, it can be considered as a bioherbicide with great potential.
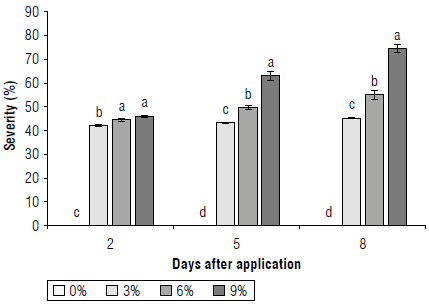
FIGURE 6 Bioherbicidal effect of the allelopathic extract of C. lineatifolia seeds on the severity of damage in Sonchus oleraceus plants. Means followed by different letters on each evaluation day indicate significant differences according to the Tukey test (P<0.05). Vertical bars of each mean indicate the standard error (n=4).
In agreement with these findings, Gil et al. (2010) report from slightly visible toxic symptoms up to death for different weeds (Senna obtusifolia, Amaranthus dubius, Rumex crispus, Brassica rapa, and Polygonum segetum) with the application of Swinglea glutinosa and Lantana camara extracts. It is important to highlight that, in the study by Gil et al. (2010), the extracts were at the maximum of 5% concentration. Blanco (2006) mentions various mechanisms of action of allelopathic agents, for example, by stimulating stomatal closure, inhibiting electron transport in photosystem II and mitochondria, decreasing ATP synthesis, affecting cell membranes, and degrading auxins, among other effects. In C. lineatifolia, the effects are unknown. It is not known how the application affects the plant. Possibly, the application directly affects chlorophylls because it generates yellowing or chlorosis. It could also greatly affect cell membranes because the effects it generates appear very fast with typical symptoms such as necrosis. Among the chemical herbicides, those that cause these symptoms, include membrane disruptors and photosynthesis inhibitors (Zimdahl, 2018). In addition, it is still not known if the effects are selective, but perhaps it is contact because the damage was localized (Fig. 5). In T. officinale, the extract of С. lineatifolia affected parameters of chlorophyll fluorescence (Cabeza-Cepeda et al., 2021) indicating that this bioherbicide can affect the photosynthetic process. In turn, the allelochemicals can be more biodegradable than traditional herbicides, but they can also have harmful effects on crops. For this reason, it is necessary to carry out more studies before adopting commercial use (Singh et al., 2003; Ma et al, 2006).
The results obtained are consistent with Stefanello (2016), in which allelopathic substances have been studied to minimize the application of agrochemicals, such as herbicides, that in the long term can minimize the negative effects on the environment and human health. Thus, Oliveros-Bastida (2008) mentioned a great diversity of substances with allelopathic potential. For phytotoxic evaluation, interest can be focused on the base molecules for the development of herbicides having around 100 molecules from each of the following groups or substances: terpenes, coumarin, benzoquinones, and alkaloids. This same author writes that the development of the use of herbicides from natural sources in the agrochemical industry has been very limited. With С. lineatifolia the first step is being taken, and other subsequent studies are necessary to make it viable as a bioherbicide on a commercial level.
Conclusions
The ethanolic extract of Campomanesia lineatifolia seeds inhibited the germination of Sonchus oleraceus seeds. With the foliar application, an incidence of 100% control was observed with and any of the concentrations evaluated showed symptoms of chlorosis and necrosis. The severity of damage was observed starting from d 2 after application, indicating that the bioherbicidal effect is rapid. At any concentration of the extract, symptoms of damage were observed in the Sonchus oleraceus plants, and, although it was not possible to cause the death of the plants, the ethanolic extract from the seeds of С. lineatifolia has bioherbicide activity on S. oleraceus.














