Introduction
The pineapple (Ananas comosus var. comosus) is the third most cultivated tropical fruit in the world, and Brazil is the third largest producer with 2.2 million t in an area of 62,116 ha (FAO, 2018).
The semi-arid zones in the world occupy around 15% of the global land surface, including the hot and cool semiarid regions. These zones are characterized by low average annual rainfall which corresponds to between one-fifth and one-half of the potential evapotranspiration. Hot semi-arid regions have a mean annual temperature above 18°C (Scholes, 2020); therefore, the Brazilian semi-arid zone is classified as hot semi-arid.
As a plant with slow vegetative growth, small size and superficial root system, the pineapple suffers intense competition with weeds, especially in the first months after planting. This contributes to delay the development of the crop and reduces productivity and quality of the fruits (Maia et al., 2012; Maia et al., 2018).
Weeds compete with agricultural crops for water, light and nutrients, and can release allelopathic substances that inhibit crop growth (Zimdahl, 2008; Ghanizadeh et al., 2014; Maia et al., 2018). Additionally, they can host pests and diseases, thus, hindering crop management and harvesting as well as impairing the quality of the marketable product (Swanton et al., 2015; Mureithi et al., 2017; Ocimati et al., 2018).
The degree of weed interference depends on the weed community, environment and the period and time of the crop's coexistence with weeds. In this sense, it is essential to identify the species present in the cultivation areas and determine the main periods of interference, indicating the appropriate time to carry out weed control in the fields (Zimdahl, 2008; Swanton et al., 2015; Marques et al., 2017).
The knowledge of the diversity of weeds in the cultivation areas is the basis for the elaboration of a weed control proposal. In addition to the identification of weed species, a survey needs to include a quantitative analysis of these species, i.e., a phytosociological study or method (Braun-Blanquet, 1979; Swanton et al., 2015). This method provides data that are specific to the species present, such as frequency, density, and abundance, and their relationship with the whole weed population. This tool allows making many inferences about the weed in question to define what will be done, how and when.
A phytosociological study for weed management is justified as infestation conditions are varied and the management possibilities are diverse (Sarmento et al., 2017; Santos et al., 2019) since the establishment and dynamics of a weed community depend on edaphoclimatic conditions, crop practices, seed bank, etc. (Adegas et al., 2010; Swanton et al., 2015; Korres et al., 2019). As phytosociological studies in irrigated crops in the hot semi-arid region showing weed community fluctuations along the year are scarce, especially in the pineapple crop, the objective of this study was to identify the dynamics of the weed community during pineapple growth in a semi-arid climate region of Brazil.
Materials and methods
The experiment was carried out in the municipality of Janaúba, MG, under the geographical coordinates of 15°43'48'' S, 43°19'23'' W and 533 m a.s.l. The region has "Aw" climate (Sá Júnior et al., 2012). The climatic conditions during the period of the experiment are shown in Figure 1.
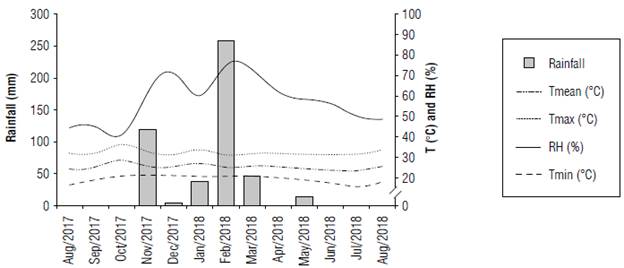
FIGURE 1 Rainfall, minimum, maximum, and mean temperatures (T, °C) and relative humidity (RH, %) during the experiment.
Weeds were collected 60, 120, 180, 240, 300, and 360 days after planting (DAP) of pineapple, in a plot composed of three pineapple cultivars (Perola, IAC Fantastic, and Smooth Cayenne) planted in double rows spaced 1.2 x 0.3 x 0.20 m, totaling 66,666 plants ha-1. The three cultivars occupied the same proportion in the sample plot.
The area was prepared by plowing at a depth of 0.40 m with two harrowings, furrowing and pre-planting fertilization with chemical and organic fertilizers. Before planting, a composite soil sample from the 0-20 cm layer was collected to determine the soil chemical properties.
The soil of the area was an eutrophic Red Latosol (Oxisol), medium/clayey texture (EMBRAPA, 2006), and the physical and chemical properties in the 0-20 cm layer were as follows: pH(H2O) = 6.5; organic matter = 15 g kg-1; P = 8 mg dm-3, K = 374 mg dm-3, Ca2+ = 3.2 cmolc dm-3; Mg = 1.2 cmolc dm-3; Al3+= 0 cmolc dm-3; H+Al = 1.4 cmolc dm-3; Zn = 1.5 mg dm-3; Fe = 23.4 mg dm-3; Mn = 33.3 mg dm-3; Cu = 0.9 mg dm-3; B = 0.3 mg dm-3; sum of base cations (SB) = 5.5 cmolc dm-3; base saturation (V) = 80%; aluminum saturation (m) = 0%; effective cation exchange capacity (CEC) = 5.5 cmolc dm-3; total CEC = 6.8 cmolc dm-3 and remaining phosphorus (P- rem) = 33.3 mg L-1.
The crop was planted in August 2017 at a depth of 0.20 m and, after furrowing, a micro-sprinkler irrigation system was implemented. After the adjustments and adaptations of the irrigation system, preliminary tests were carried out to determine the flow rate of the micro-sprinklers and the water distribution uniformity coefficient. Slip, slip-sucker, and sucker propagules were used for planting with the same kind of propagule in the same plot to maintain crop uniformity.
For weed collection, the standard method of the square inventory (0.5 m x 0.5 m) was employed, randomly thrown once in the useful area of each plot. The square was thrown three times per collection, collecting all plants as described by several authors (Curtis & Mcintosh, 1950; Odum, 1971; Braun-Blanquet, 1979; Swanton et al., 2015). Only the weed shoots were collected. The identification of the species in each square was carried out by comparison, according to the classification of Lorenzi (2008) and quantified by family, genus and species, with later determination of the phytosociological values.
The samples of each species were then packed in paper bags and put into a forced air circulation oven (model TE 394/3, Tecnal, Piracicaba, Brazil) at 65°C for 72 h, for later weighing of the dry matter mass on a precision scale, with the result expressed in g. The total dry matter of the weed community was used to adjust a growth model and estimate the absolute and relative growth rate of the weed community as a function of growth sampling dates in the pineapple crop (Hunt, 2012; Soltani & Sinclair, 2012).
The number of individuals per species in each plot and the total number per collection were determined. From the identification and counting of species, we carried out the calculations and descriptive analysis of the following phytosociological variables: frequency (FR), density (DE), abundance (AB), relative frequency (FRR), relative density (DER), relative abundance (ABR), relative dominance (DOR), importance value index (IVI) and coverage value index (CVI) (Mueller-Dombois & Ellenberg, 1974; Braun-Blanquet, 1979; Tuffi Santos et al, 2004).
The relative indexes were used to calculate the importance value indexes expressed as constants. To perform the calculations, the following formulas were used:
FR = number of squares containing the species / total number of squares obtained;
FRR = FR of the species x 100 / total frequency of all species;
DE = total number of individuals per species / total area occupied by the squares;
DER = DE of the species x 100 / total density of all species;
AB = total number of individuals per species / total number of squares containing the species;
ABR = AB of the species x 100 / total abundance of all species;
DOR = biomass of the species / E of total biomass of all species x 100;
IVI = FRR + DER + ABR;
CVI = DOR + DER.
All results were presented using descriptive statistics done with Microsoft Excel. The graphs were made using the software Sigma Plot 12.5 Demo Version.
Results and discussion
Throughout the pineapple growth cycle, the weed community obtained by the surveys showed a distribution of 22 species and 12 families. Among the species, a population of 68.2% of dicotyledonous plants and 31.8% of monocotyledonous plants was found (Tab. 1). As for the carbon fixation process, 54.6% were plants with C3 metabolism and 45.4% were with C4 metabolism. No plants with CAM metabolism were observed.
TABLE 1 Botanical and photosynthetic classification of weeds found during pineapple cultivation in the semi-arid climate region of Brazil.
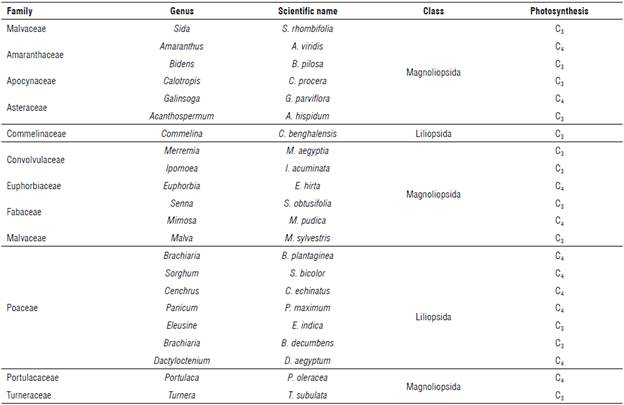
Regarding the number of species, among the dicots, the families Amaranthaceae, Asteraceae, Convolvulaceae, Fabaceae and Malvaceae stood out. These families corresponded to 45.5% of the identified species. Among the monocotyledonous plants, the family Poaceae stood out, with five species, equivalent to 22.7% of the identified species (Tab. 1). Sarmento et al. (2017) cultivated pineapple under similar climatic conditions in the spring-summer period of Brazil and identified ten weed species distributed in nine genera and eight families, with the families Euphor-biaceae and Poaceae standing out, with two individuals each. In the autumn-winter period, nine species, seven genera and six families were identified, with Euphorbia-ceae and Poaceae standing out again with three and two individuals, respectively.
Model et al. (2008) reported that 40 weed species were identified in an area of pineapple cultivation in Maquiné (RS, Brazil), a region with a humid subtropical climate. The authors highlighted the presence of the families Asteraceae (27.5%), Poaceae (25%), Cyperaceae (7.5%), Fabaceae (7.5%), Apiaceae (5%) and others with 2.5% each. In the following year, Model and Favreto (2009) identified 74 weed species belonging to 25 botanical families, with the families Poaceae (23%), Asteraceae (28%), Cyperaceae (5%), and Fabaceae (5%) standing out. Similar results were found in pineapple areas located in the humid tropics of India (Kerala) (Girija & Menon, 2019).
The high number of species and families shows the great botanical diversity of the weed flora with the potential to compete with the pineapple. The favorable climate, soil productive potential, pH, and nutrient corrections are factors that can influence the diversity of species and the development of weeds (Model & Favreto, 2009). In addition to these factors is the use of irrigation, which allows for the germination and growth of weeds to occur in all months of the year.
The Asteraceae and Poaceae families have been commonly reported and found in several weed studies (Jakelaitis et al., 2003; Erasmo et al., 2004; Murphy et al., 2006; Duarte et al., 2007; Maia et al., 2018). This is certainly because they show high dissemination and colonization of different environments (Pedrotti & Guarim Neto, 1998).
Most of the species of the family Poaceae are perennial and produce large amounts of seeds, which considerably increases their dissemination power and the colonization of different types of environments, even under adverse conditions (Holm et al., 1991).
The species of the family Asteraceae have similar characteristics and are easily established in different environments, being the first weeds in the cultivation area after soil preparation (Lorenzi, 2008). The species of this family are considered among the most infesting plants of annual and perennial crops (Holm et al., 1991; Zimdahl, 2008). The identification of weed species is important and necessary, as each species has the potential to establish itself in the area and can interfere differently, depending on the crop (Korres et al, 2019).
The dry matter of the weed community increased during the entire survey period. There was an exception at 180 d, with dry matter of 1,780.58 g m-2, i.e., slightly lower than the previously evaluated period. As a result, the maximum accumulated dry matter (3,436.77 g m-2) of the weed community was reached 360 DAP (Tab. 2). This is probably due to the greater adaptation and competition potential of these species compared to the pineapple plants.
TABLE 2 Dry matter (DM, g m2) and number of individuals per species (NIS) of weeds found during pineapple cultivation in the semi-arid climate region of Brazil.
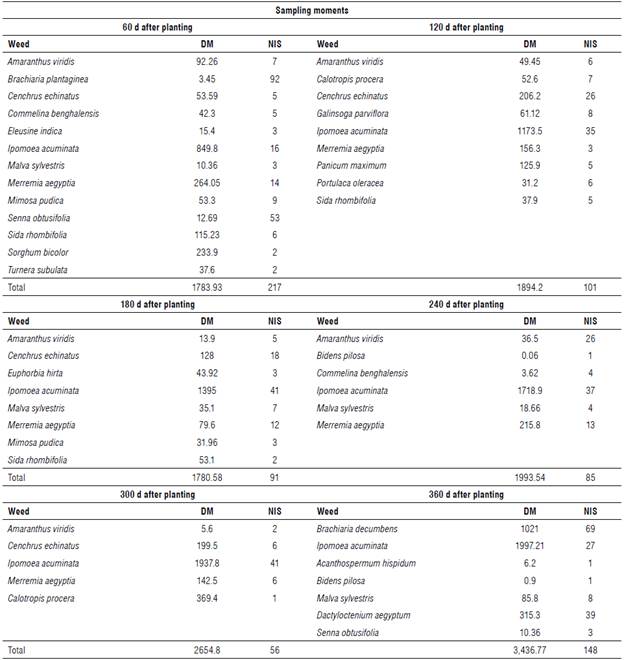
Weed species can harm the crop by competing for the essential resources for plant development (water, light, and nutrients), especially nutrient extraction. Considering the highest value of dry matter obtained at 360 DAP (3,436.77 g m-2 = 34.4 t ha-1), it can be estimated that 515.5 kg of N (1.5%), 61.85 kg of P (0.18%), 580.79 kg of К (1.69%), 154.6 kg of Ca (0.45%), 164.96 kg of Mg (0.48%), and 68.73 kg of S (0.20%) are extracted from the soil by weeds at the expense of pineapple plants.
Those values were estimated based on a study by Souza et al. (1999), who evaluated the levels of macro and micro-nutrients and the C:N ratio of various weed species of the families Commelinaceae, Poaceae, Cyperaceae, Amaran-thaceae, Compositae, Convolvulaceae, Euphorbiaceae, Malvaceae, and Rubiaceae.
The accumulation of dry matter in the weed community over the periods of evaluation showed exponential growth, with an increase in the amount of dry matter accumulated as a function of time. The highest dry matter value (3,436.77 g m-2) was observed on the last collection date. An increment of 0.0128 g per d was observed from the sampling times of the weed community in the pineapple crop (Fig. 2A).
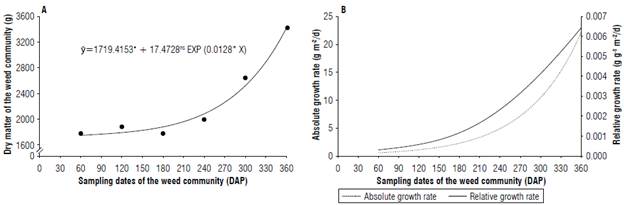
FIGURE 2 A) Dry matter and B) absolute growth rate (g nr2/d) and relative growth rate (g g1 nr2/d) of the weed community as a function of growth sampling dates in the pineapple crop. DAP - days after planting.
From the adjusted model, the absolute and relative growth rates of the weed community were estimated. The absolute growth rate (AGR) is a physiological index used to obtain the average growth speed over the observation period (Hunt, 2012; Soltani & Sinclair, 2012). On the other hand, the relative growth rate (RGR) expresses the increase in dry matter per unit of the initial weight over a period of time (g g"7d) (Hunt, 2012; Soltani & Sinclair, 2012).
The relative and absolute growth rates showed the same exponential behavior observed for dry matter accumulation (Fig. 2B). The maximum observed AGR value was 22.29 g m-2/d, while the maximum observed RGR was 0.00644 g g-1 m-2/d, both observed on the last collection date.
The growth rate is an important feature to describe the ecological strategies of plants. Weeds, in general, show rapid initial growth, allowing them to absorb mineral nutrients and develop in environments without limitations, thus, being able to stabilize in the environment (Ravindra et al., 2008;Korres et al, 2019).
In the analysis of plant growth, the absolute growth rate usually shows an increase until it reaches a peak, with a subsequent fall. The relative growth rate, in turn, decreases over the time of collection. The higher the values of this rate and the longer the time it remains high, the greater the competition capacity of the species in question. Therefore, the weed community showed great competitive capacity with the studied species (pineapple) and that this capacity increased over time. This indicates that, the longer the pineapple is subjected to weed competition, the greater the competition capacity of these species.
During the pineapple growth period, the species that showed the best carbon fixation capacity, in terms of dry matter, were Ipomoea acuminata, Merremia aegyptia, and Brachiaria decumbens (Tab. 2). The other species collected showed the lowest number of individuals and lower dry matter values than those previously mentioned. However, these values can be considered representative since the capacity to extract resources from the soil tends to increase possibly with the higher number of individuals.
Ipomoea acuminata and Merremia aegyptia have a climbing habit, which can make certain growing practices and pineapple harvesting difficult. Brachiaria decumbens, being a C4 metabolism plant, tends to be more efficient in the use of scarce resources, especially water, considering the semi-arid climate conditions (Zimdahl, 2018).
At 60 DAP, 217 weed specimens were identified (Tab. 2), with Brachiaria plantaginea and Senna obtusifolia having the highest number of individuals per species (NIS) and, consequently, the highest relative density (DER), relative abundance (ABR) and importance value index (IVI) (Fig. 3). However, Ipomoea acuminata and Merremia aegyptia showed higher dry matter compared to the other species.
When the pineapple reached 120 and 180 DAP, 101 and 91 weed specimens were observed, respectively, with the largest number of individuals and amount of dry matter observed for the species I. acuminata and Cenchrus echinatus (Tab. 2). For these two species, higher values of DER, ABR and IVI were also observed (Fig. 3). However, the highest relative frequency (FRR) was observed only for I. acuminata, 180 DAP. At 240 and 300 DAP, there was a reduction in the number of specimens. However, I. acuminata remained with a larger number of individuals and showed higher dry matter in this period. This behavior remained similar for FRR, DER, ABR, and IVI (Fig. 3).
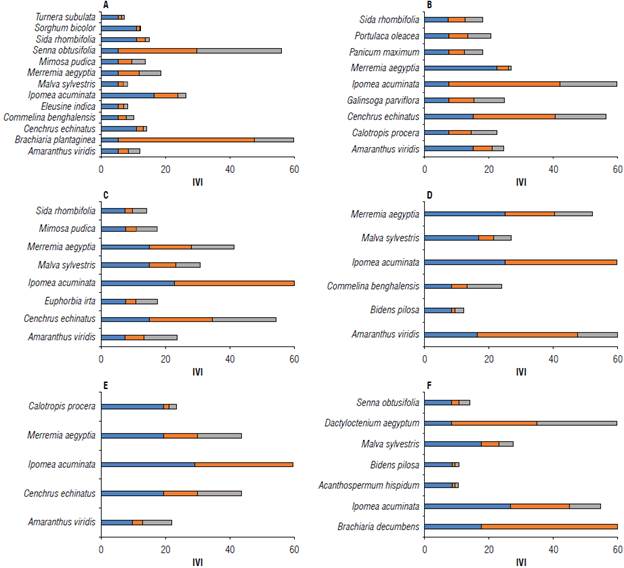
FIGURE 3 Importance value index (IVI) of the main species of weeds collected during the development of pineapple, in days after planting (DAP), cultivated in the semi-arid climate region of Brazil. Blue bar = relative frequency (FRR); orange bar = relative density (DER); gray bar = relative abundance (ABR). A) 60 DAP; B) 120 DAP; C) 180 DAP; D) 240 DAP; E) 300 DAP; F) 360 DAP.
Sarmento et al. (2017) observed higher frequency (FR) values for the species Euphorbia heterophylla, Cynodon dactylon, Dactyloctenium aegyptium, and Amaranthus hybridus in the spring-summer period of Brazil. The greatest distribution in the autumn-winter period was observed in the species Euphorbia hirta, Amaranthus hybridus, Euphorbia heterphylla, and Portulaca oleracea. Unlike this study, the same authors found that the species that showed the highest IVI values were Cyperus iria, Cynodon dactylon, Euphorbia heterophylla, Amaranthus hybridus, Euphorbia hirta, and Boerhavia diffusa. This is because, although the study was conducted under similar climatic conditions, the spatial variation in the distribution of weed species was large, confirming that other factors influence the diversity of the weed community.
Brachiaria decumbens and Dactyloctenium aegyptum were found only at 360 DAP, while I. acuminata was identified at all survey times (Tab. 2). A greater number of individuals at 360 DAP was observed in the species B. decumbens, D. aegyptum, and I. acuminata, respectively, as well as the greater FRR, DER, ABR, and IVI (Fig. 3).
The highest weed density during the pineapple development cycle was verified at 60 DAP, when 217 individuals per m2 were counted. The lowest density was observed at 300 DAP, with 56 individuals per m2 (Tab. 2). According to Radosevich et al. (2007), as the density and development of weeds that germinate and emerge at the beginning of the crop cycle increases, interspecific and intraspecific competition intensifies, so that more developed weeds become dominant, and the rest are suppressed or die.
The diversity and density of weeds were higher in the first days after planting, i.e., crop still establishing itself. At this stage, the plant has not yet reached the ideal height of the shoot; therefore, its potential to shade the soil is low, allowing weeds to receive sunlight. Additionally, the pineapple has a slow growth pattern, which makes it uncompetitive against weeds.
At 360 DAP, weed diversity was reduced, as in other survey periods, compared to the first 60 DAP. This probably occurred due to competition between weeds, as some are more aggressive than others, resulting in reduced emergence and/ or growth of weeds.
The species that stand out from the others generally have high seed production (to supply the soil seed bank), combined with other mechanisms, such as dispersion, longevity, and dormancy for a long period. Species developing these mechanisms can survive even under adverse conditions (Zimdahl, 2008; 2018; Korres et al., 2019).
Regarding relative dominance (DOR), I. acuminata stood out in relation to the other species in all survey periods, except for 300 DAP, when M. aegyptia showed the highest DOR value (Tab. 3). At 360 DAP, the species I. acuminata (58.11), B. decumbens (29.70), and D. aegyptum (9.17) stood out. These same species produced a higher number of individuals per species and higher dry matter in the same period.
TABLE 3 Relative dominance (DOR) and weed cover value index (CVI) during the pineapple cultivation cycle in the semi-arid climate region of Brazil.
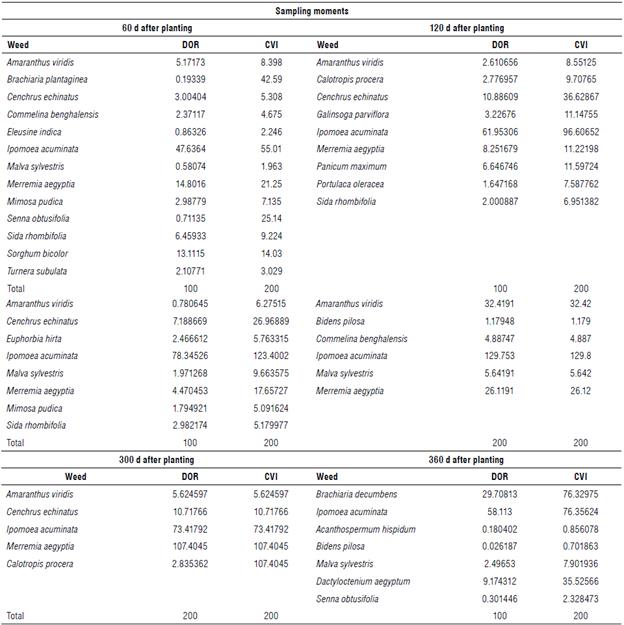
The species that stood out in relation to the coverage value index (CVI) were I. acuminata and B. plantaginea (55.01 and 42.59) at 60 DAP. Ipomoea acuminata and C. echinatus showed higher CVI values (96.60 and 36.62, respectively) at 120 and 180 DAP. Merremia aegyptia showed CVI equal to 17.65 at 180 DAP. In the last survey period, 360 DAP, I. acuminata (76.35), B. decumbens (76.32), and D. aegyptium (35.52) showed higher CVI values compared to the other weeds. According to the CVI, the species I. acuminata covered most of the study area during the collection periods.
Known as a common morning-glory, the species I. acuminata was present on all collecting dates. This plant has a climbing growth habit, as do the species of the Merremia genus, which was also present in almost all surveys but in a smaller number. As these weeds are herbaceous climbing plants that reproduce by seeds and, in general, prefer tilled, fertile soils with good humidity, they can establish easily. As for competition for nutrients, the common morning-glories can be great extractors, as observed by Souza et al. (1999) in the sugarcane crop.
Conclusions
The weed community found in irrigated pineapple cultivation under semi-arid climate conditions was composed mostly of species belonging to the families Amarantha-ceae, Asteraceae, Convolvulaceae, Fabaceae, Malvaceae, and Poaceae. The highest diversity of weed species was found 60 d after planting. The diversity of weed species decreased over the pineapple growth cycle. The species Ipomoea acuminata was present throughout the growth of the pineapple and showed a higher index of importance value in most periods evaluated.














