Introduction
Clubroot disease is caused by Plasmodiophora brassicae Woronin, a soilborne protozoan. In Latin America, this disease has been reported from Mexico, Costa Rica, Guatemala, Bolivia, Venezuela, Ecuador, Peru, Chile, Brazil, and Colombia; however, studies reporting the disease severity and economic losses it causes are not available (Botero et al., 2019). Clubroot reduces yield in vegetable crops of the Brassicaceae family that, in 2017 occupied 2600 ha in Colombia (3.5% of the cultivation area in vegetable crops in the country) (MADR, 2018). Disease symptoms are observed as galls in the plant roots that impede nutrients and water uptake and cause growth delay, wilting, chlorosis, and even plant death when symptoms are severe (Dixon, 2009).
The disease cycle is divided into three main stages. During the primary infection, resting spores in soil detect root exudates of a host plant and induces their germination into a primary zoospore that swims towards the plant root hairs. Once in the root hairs, penetration occurs; and a primary plasmodium develops. This plasmodium will later cleave into a zoosporangium that produces secondary zoospores that are responsible for the secondary infection. During the secondary stage, cells of the root cortex are infected; and the disease symptoms become visible. The last stage happens when resting spores are produced in the root galls to be later released into the soil. Those resting spores will serve as primary inoculum for future infections (Kageyama & Asano, 2009).
Clubroot management is quite difficult, mainly because of the production of resting spores that can survive in soil in the absence of a host for up to 17 years (Wallenhammar, 1996). However, their average lifespan is around five to six years (Hwang et al, 2015). This is one of the main features that makes clubroot disease one of the major threats to cruciferous crop production around the world, including Colombia (Jaramillo & Díaz, 2006; Dixon, 2009).
Incidence and severity of diseases are modulated by the disease triangle components (host, environment, and pathogen) (Agrios, 2005). Among the environmental conditions affecting clubroot development, the most important are temperature, soil moisture, and soil properties. The most studied are soil pH, boron, and calcium contents. The optimum pH range for clubroot development has previously been determined to be between 5 and 6.5 (Webster & Dixon, 1991b; Narisawa et al., 2005; Niwa et al., 2007, 2008; Gossen et al., 2013; Rashid et al., 2013). An increase of concentration of both the nutrients calcium and boron is related to a reduction in the primary infection of the pathogen and plasmodia dehiscence (Webster & Dixon, 1991a, 1991b). Soil moisture is regarded as one of the most important factors affecting clubroot development, with disease incidence and severity increasing together with moisture levels (Samuel & Garrett, 1945; Hamilton & Crête, 1978; Dobson et al., 1982; Narisawa et al., 2005). Finally, previous reports have found that the optimal conditions for clubroot development include a temperature between 20 and 25°C, a soil pH between 5 and 5.6, and an inoculum density of 106 resting spores per plant (Sharma et al, 2011; Gossen et al., 2012, 2013).
While the planting of resistant cultivars is the most efficient and convenient method for clubroot management, intensive cropping of clubroot resistant cultivars (CR) exerts significant selection pressure on the pathogen (Holtz et al., 2018). This pressure can result in shifts in the virulence of pathogen populations, favoring the emergence of pathotypes that can break or overcome resistance, as has already been observed in canola and Chinese cabbage in Japan, Canada, and Europe (Kuginuki et al., 1999; Diederichsen et al., 2014; Orgeur et al., 2016; Strelkov et al., 2016).
Understanding the spatial patterns of pathogen populations or diseased plants is crucial to design disease management strategies (Madden et al., 2007). In Colombia, clubroot research is scarce and has been focused mainly on disease management (Velandia et al., 1998; Botero et al., 2015; Botero-Ramírez et al., 2016). Furthermore, currently, clubroot prevalence in Colombia and the relationship of its occurrence in the main cruciferous crops (cabbage (Brassica oleracea var. capitata), broccoli (B. oleracea var. italica) and cauliflower (B. oleracea var. botrytis)) with field management practices, soil properties and climatic characteristics are unknown.
Given the lack of knowledge on clubroot prevalence in Colombia and the relationship of the disease occurrence with soil properties, climate, and crop management strategies, this research sought to achieve two main objectives: i) to determine the disease prevalence in the most important regions where cruciferous crops are grown in Colombia; ii) to evaluate the correlation between the soil properties, climate characteristics, and crop management strategies with the disease occurrence. This knowledge is needed to understand the impact of production practices of cruciferous crops on clubroot disease to outline more accurate disease management strategies.
Materials and methods
In total, 127 fields were visited in February and March of 2017 to establish the prevalence of clubroot throughout the main productive regions of cruciferous crops in Colombia. The fields were located in the departments of Cundinamarca, Antioquia, Nariño, Boyacá, Valle del Cauca, Norte de Santander, Caldas, and Cauca. Caldas was included because it was the first department where clubroot was reported in 1969 (Torres, 1969). The number of samples collected in each department was defined based on the cropped area in cabbage, broccoli, and cauliflower in 2016 (MADR, 2016) (Tab. 1).
TABLE 1 Cultivated area of cruciferous crops in the most productive departments of Colombia and the number of fields visited in each department.
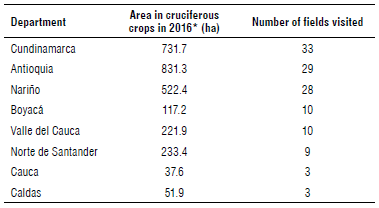
*The data presented are based on statistics of production from the Ministry of Agriculture and Rural Development (MADR, 2018).
Clubroot infestation
A field was determined as clubroot infested either by direct observation of typical symptoms (galling on roots) in cruciferous crops or weeds or after being reported by the farmer. When the field was with cruciferous crops at the time of the visit, plants were evaluated for the presence of symptoms; when a different crop was grown, cruciferous weeds were assessed.
When cruciferous crops were growing, twenty plants were extracted and assessed for the presence of root galls, ten were evaluated at the field entrance, and ten more following the "W" pattern sampling. When a different crop was growing, nine points were assessed following the "W" pattern sampling for the presence of cruciferous weeds, and when present, those were removed and evaluated for the presence of typical clubroot symptoms. In either case, once the disease symptoms were observed the sampling was stopped, and the field was set as clubroot infested. In those cases, were the farmer confirmed previous observations of the disease symptoms, plants were also evaluated at the patches where clubroot had been observed before.
Soil samples
At each sampling site, a composite soil sample of 500 g was collected from the top 20 cm of the soil profile. At the central point of the "W" a metal cylinder with unperturbed soil was collected for bulk and particle density estimation. Chemical and physical analyzes of the samples were performed at the Soil and Water Laboratory of the Faculty of Agricultural Sciences at the Universidad Nacional de Colombia.
Crop management information
Information regarding the management of the fields and clubroot disease was obtained by surveying the farmers in the visited fields. The farmers were interviewed if they were familiar with clubroot symptoms; if they were not familiar with clubroot symptoms, photographs of typical symptoms of the disease were shown, and they were asked again if they had observed them before.
On management strategies, farmers were asked about the period during which the farmer had been growing the field, the cultivated area, the rotation scheme, the cruciferous cultivars planted, the propagation strategy, the machinery used and its provenance, the type and application frequency of liming materials and compost, and harvest residue management. In total, 98 farmers were surveyed, since at some places it was impossible to contact the field owner or worker.
Climatic information
Climatic information was obtained from the closest IDEAM weather station to the sampling point. The dataset consisted in the historical normalized data from 1982 to 2010 (IDEAM, 2014). Analysis included average, maximum and minimum temperature, relative humidity, monthly precipitation, and number of rainy days per year.
Statistical analysis
Data analysis was performed using the SAS software (Version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA).
Point biserial correlation analysis were done to correlate continuous (climatic variables, soil properties) and dichotomic variables (crop management practices and disease infestation) (Kornbrot, 2014) using the CORR procedure.
Results
Description of cruciferous crops in Colombia
Cruciferous crops were grown in 80 of the 127 surveyed fields. In those the main identified crops included green cabbage (64 fields representing 80% of the fields grown in cruciferous crops), red cabbage (5 fields representing 6.25% of the fields grown in cruciferous crops), broccoli (6 fields representing 7.5% of the fields grown in cruciferous crops), and cauliflower (5 fields representing 6.25 % of the fields).
The most commonly grown green cabbage cultivars were the susceptible hybrids 'Delus' (Semillas Arroyave, 2006), and 'Globe Master' (Agroglobal S.A, 2022a), and the CR hybrid 'Tekila' (Syngenta, 2016). Those hybrids were grown in different regions, 'Delus' was cultivated mainly in Cun-dinamarca and Boyacá, while 'Globe Master' was mostly grown in Caldas and Norte de Santander. On the other hand, the CR hybrid 'Tekila' predominated in Antioquia.
In all other departments, farmers do not know the name of the variety they were growing.
None of the other cruciferous species showed a clear pattern in the department where they were grown. Of the 25% of farmers cultivating red cabbage, 67% of the farmers growing broccoli, and 60% of those growing cauliflower did not know the name of the variety they grew.
Half of the farmers cultivating red cabbage grew the hybrid 'Ruby King' (Agroglobal S.A, 2022b) and 25% of them grew the hybrid 'Sombrero' (Bejo Eurosemillas, 2022). For broccoli, 33% of the farmers cultivated the hybrid 'Legacy' (Bayer, 2022), and, for cauliflower, 40% of the farmers employed the hybrid 'Skywalker-Fl' (Bejo, 2022). It must be pointed out that none of the cropped varieties of red cabbage, broccoli or cauliflower are clubroot resistant.
Cruciferous crops are grown in small areas; the national average size of the fields was 3 ha. The size of the fields was different among departments. Antioquia, Cundinamarca, Valle del Cauca, and Nariño were the only departments with fields larger than 1 ha with average sizes of 7.1, 3.3, 1.8 and 1.6 ha, respectively.
Production of cruciferous crops in the country had high agroclimatic variability and these are allocated at altitudes between 1600 and 3000 m a.s.l. From the surveyed field, 10% of the fields were at altitudes between 1600 and 2000 m a.s.l., 20% were between 2000 and 2500 m a.s.l., and the remaining 30% were between 2500 and 3100 m a.s.l. In Cundinamarca, Boyacá, Nariño, and Norte de Santander (half of the visited crops) cruciferous crops were allocated at altitudes between 2500 to 3600 m a.s.l. In Antioquia and the Norte de Santander cruciferous crops were at altitudes between 2000 and 2500 m a.s.l. In Cauca, Valle del Cauca and Caldas, the fields were at altitudes between 1674 and 2343 m a.s.l.
Clubroot prevalence in Colombia
The prevalence of clubroot was established within the departments where most of the cruciferous crops are grown in the country (Fig. 1). Those fields where the disease symptoms were observed in any host plant or where the farmer reported its occurrence in previous cycles of cruciferous crops were reported as clubroot infested. Clubroot was present in 53.6% of the sampled fields; from these, 48.8% were fields where the disease was observed by the researchers. The remaining 4.8% were assumed as positives as the farmer reported to have observed the disease in previous crop cycles. The disease was observed in all visited departments with the exception of Nariño (Figs. 1 and 2).
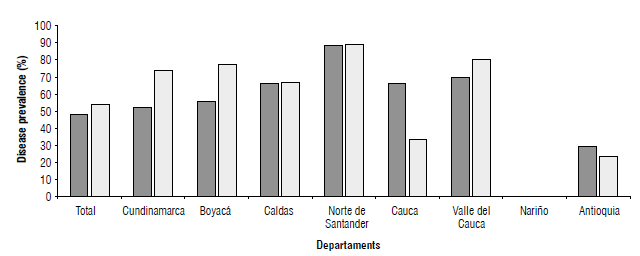
FIGURE 1 Clubroot prevalence In the main productive departments of cruciferous crops In Colombia. The graph shows the percentage of fields where clubroot symptoms were observed In any susceptible host (dark bars) or where farmers reported observation of clubroot symptoms In previous cycles of cruciferous crops (white bars). Percentages were estimated using a total of 127 fields visited in 2017.

FIGURE 2 Clubroot prevalence in the main productive areas of cruciferous crops in Colombia. The map presents the departments were a survey for clubroot disease was conducted in 127 fields; the color cyan shows the only department where clubroot symptoms were not observed nor reported; in red the departments where clubroot symptoms were observed and/or reported.
In Nariño, Boyacá, and Caldas, most of the farmers were not familiar with the disease symptoms (data not shown). In the municipalities of Sogamoso (Boyacá) and Popayán (Cauca), plants with typical clubroot symptoms were observed in fields of farmers who were not familiar with the disease and could not recognize its symptoms. Furthermore, they attributed yield losses, and symptoms such as wilting and reduction in the crop (caused by plant death) to different stresses such as water deficit and nutritional deficiencies. In Antioquia, the disease symptoms were not observed in green cabbage.
According to the farmers, clubroot was observed the first time around 2001 in Cundinamarca, 2011 in Boyacá, and 2013 in Norte de Santander. It was impossible to determine the approximate year when the disease was observed for the first time in Antioquia, Caldas, Valle del Cauca, and Cauca.
Relationship between the soil, environmental characteristics, and clubroot infestation
A correlation between clubroot infestation, soil characteristics, and environmental variables was established (Tab. 2). From the evaluated variables, calcium, phosphorus, boron, copper, soil pH and the effective cation exchange capacity (ECEC) were positively correlated with the disease infestation (Tab. 2). Those results show that as levels in these variables increase, the odds of observing the disease in a field increases as well. However, aluminium content in soil and the average of rainy days per year showed a negative correlation with clubroot infestation. This implies that the odds of finding the disease in a field are reduced as the aluminium content in soil and the number of rainy days per year increase.
TABLE 2 Point biserial correlation of clubroot infestation and soil properties or environmental characteristics in the main productive areas of cruciferous crops in Colombia.
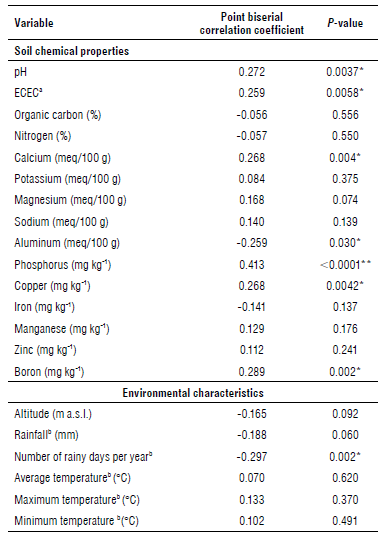
a Effective Cation Exchange Capacity.
b Annual average of the historical normalized data from 1981-2010.
The asterisks (*) show the variables which are correlated with clubroot infestation (P<0.05).
Relationship between crop management and clubroot infestation
Analysis showed that the probability of finding a clubroot-infested field is higher whenever cruciferous crops are included in the rotation scheme, and these chances are reduced when CR cultivars are included in the rotation scheme (Tab. 3).
Soil and climatic characteristics and their relationship with the clubroot infestation
Only the soil pH, the ECEC, the aluminium, phosphorous, calcium, boron and copper contents in soil and the number of rainy days per year correlated with clubroot infestation. The pH of the sampled soils was between 4.45 and 7.75. In Cundinamarca, soil pH was between 4.66 and 7.07, in Boyacá it was between 5.11 and 7.7, between 4.75 and 6.93 in Caldas, between 6.12 and 7.75 in Norte de Santander, between 4.68 and 7.72 in Valle del Cauca, between 4.45 and 6.47 in Cauca, between 4.85 and 7.33 in Nariño, and between 4.85 and 7.33 in Antioquia. The ECEC was highly variable, ranging from 1.03 to 85 meq 100 g-1, and Cundinamarca and Boyacá had the highest averages (33 and 30 meq 100 g-1, respectively). Calcium, aluminium, phosphorus, copper, and boron contents in soil had average values of 17.22 meq 100 g-1, 0.76 meq 100 g-1, 21.99 mg kg-1, 2.23 mg kg-1, and 0.59 mg kg-1, respectively. The national average of number of rainy days per year was 149, and departmental averages were 135 d in Boyacá, 180 d in Caldas, 171 d in Norte de Santander, 213 d in Cauca, 161 d in Valle del Cauca, 195 d in Nariño, and 217 d in Antioquia.
Discussion
This research reports on the first clubroot survey conducted in Colombia, and to our knowledge in Latin America. The research allowed the estimation of the disease prevalence in the main productive areas of cruciferous crops in Colombia. It also assessed the correlation between clubroot infestation and soil and climatic characteristics, and crop management strategies.
This survey confirmed that clubroot is present in all departments where cruciferous crops are grown in Colombia with the exception of Nariño. These results expand previous reports from Torres (1969) and Jaramillo and Díaz (2006), who confirmed the disease presence in Cundinamarca, Antioquia and Caldas, therefore, confirming for the first time clubroot infestation of fields in Norte de Santander, Cauca, Valle del Cauca and Boyacá. Though in Nariño disease symptoms were not observed, the department cannot be declared 'clubroot free' yet; further confirmation is required by the application of molecular techniques for P. brassicae detection in soil such as endpoint PCR and/ or qPCR (Cao et al., 2007; Rennie et al., 2011). Given the widespread presence of the pathogen in the country, it is likely that some inoculum of P. brassicae is already present in the fields of Nariño, but the inoculum densities are below the required threshold to cause visible disease symptoms under the predominant environmental conditions. Hwang et al. (2011) reported that for consistent symptoms of development under highly conductive conditions, a minimum inoculum density of 1x103 resting spores per gram of soil is required. The department either has lower inoculum densities or the environmental conditions are not conducive for symptom development.
Our results show that about half of the fields where cruciferous crops are grown in Colombia are infested with clubroot (national prevalence 53.6%). However, this estimation is likely skewed, and the percentage of infested fields is even higher. Such skewing might have been caused by Antioquia, since in this department clubroot symptoms were not found in fields grown with white cabbage since all of them were grown with the cultivar 'Tekila'. Therefore, it is likely that P. brassicae inoculum is already present in those fields, but they were reported as non-infested since disease symptoms were not observed by the researchers nor by the farmers in that department. The fields where clubroot was reported and/or observed were grown with red cabbage, broccoli, or cauliflower, given that resistant cultivars with highly commercial acceptance are not available.
A positive relationship was observed between the disease's presence and some edaphic conditions such as pH, ECEC, and aluminium, phosphorus, calcium, boron, and copper contents in soil. Calcium content in soil and pH are crucial for clubroot development (Webster & Dixon, 1991b). Previous research shows a weak correlation between clubroot and soil pH, where alkaline soils (with pH higher than 7.2) can reduce disease levels even under highly conductive scenarios (Wallenhammar, 1996; Gossen et al., 2013). These results at first glance would indicate a disagreement of previous reports with ours, since our results show that as the pH increases so does the likelihood of finding an infested field.
Conducive pH levels for clubroot development are between 5.0 and 6.5 (Webster & Dixon, 1991a; Narisawa et al., 2005; Niwa et al., 2007, 2008; Ruaro et al., 2010; Gossen et al., 2013; Rashid et al., 2013). From the collected soil samples 83.6% were acidic, from those, 76% were in the range between 5.0 and 6.5 the most favourable pH for the disease development. This might indicate that the observed pH values in this research are below or very close to the optimum required for disease development. For that reason, as the pH levels are increased the conditions for the disease development are improved, explaining the observed correlation.
As calcium and boron content in soil increased, the chance of finding clubroot in a field increased. These results differ from previous research reporting that an increment in calcium and boron concentrations cause a reduction in the number of infections and expression of the disease symptoms (Webster & Dixon, 1991a, 1991b). The effect of these nutrients over the disease symptoms is negatively correlated with the inoculum levels; and thus, under high inoculum pressure, disease reduction is diminished (Webster & Dixon, 1991a, 1991b; Gossen et al., 2014). In most of the infested fields, disease was very severe (data not shown); as disease severity is positively corelated with high inoculum densities (Murakami et al., 2002) we can presume that inoculum levels in the infested fields is high, hindering the calcium and boron effect on it.
Bhering et al. (2017) reported that low pH values and high aluminium contents in soil are favourable for clubroot development; however, our results show the opposite pattern. The negative correlation between clubroot infestation and aluminium contents in soil might be explained mainly by the negative effect of aluminium over the reduction of root branching in the host roots (Marschner, 1995); it can lower the infection probability and along with it the chance of finding the disease in a field. However, that hypothesis needs to be proved by further research.
The largest correlation coefficient with disease occurrence was for the inclusion of cruciferous crops in the rotation scheme; that agrees with reports from Gossen et al. (2014) and Dixon (2009), who state that clubroot incidence and severity increases as cruciferous crops are intensified.
Finally, a negative correlation was found in the number of rainy days per year and clubroot infestation. Bhering et al. (2017) indicates a lower chance of observing clubroot disease in areas with low precipitation and well drained soils, but fluctuations in the amount of precipitation (rainy seasons, with precipitation levels over the average, followed by drought seasons) can cause epidemics. It is also important to dig deeper into this observation, since it might be a spurious result; the departments with more rainy days per year were Antioquia and Nariño that also are the ones with the lowest number of infested fields, the first one because mostly CR cabbage is grown and the second because it might be clubroot free or the inoculum densities are low.
This research confirmed the presence of clubroot in the main productive departments of cruciferous crops in Colombia with the exception of Nariño. Based in the current methodology, Nariño appears to be clubroot-free; however, further confirmation is required. This is the first research that attempts to establish clubroot disease status in Colombia, and it becomes a starting point for the design and implementation of integrated management practices for the disease.















