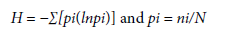Introduction
According to FAO statistics, the primary producers of chickpea worldwide are India, Turkey, and Russia, and the chickpea grain yield average is 1,038 kg ha-1 worldwide (FAO, 2021). The area under cultivation of chickpea in Iran is about 456 thousand ha, with an average grain yield of 439 kg ha-1, which is very low compared to the global average yield.
Weeds are the biggest challenge to food production worldwide and reduce crop yields due to high competitiveness (Naghib et al., 2020). The competitive capacity of chickpea is lower than other crops compared to weeds, so productivity is seriously affected by weeds (Abdulahi et al., 2012). There are different reports of weed damage to chickpea fields under the free control of weeds. Some studies reported a 92% reduction in performance and a damage rate of up to 97% (Paolini et al., 2006; Mousavi et al., 2007). In western Iran (Kurdistan), a 77.5% reduction in yield because of weed interference is estimated (Fathi et al., 2017). Another research estimates the amount of damage in weed-free control as 48.3% in Kermanshah and 66.4% in Tabriz (Mohammadi et al., 2005). Due to the long growing season and rainfall in autumn and winter, weeds are a massive problem in winter cultivation of chickpeas and sometimes heavy weed infestations can cause 88% crop failure, while in spring cultivation with plowing before planting, a large volume of weeds are controlled (Knott & Halila, 1988). Important and dominant broadleaf weeds of chickpea fields in Kermanshah were chicory (Cichorium intybus L.), bindweed (Convolvulus arvensis L.), stickywilly (Galium aparine L.), Jeweled distaff thistle (Carthamus oxyacantha M. Bieb.), and cowcockle (Vaccaria pyramidata Medik.). The narrow leaf weeds were wild barley (Hordeum spontaneum Koch.), wild oat (Avena ludoviciana Durieu.), and bermuda grass (Cynodon dactylon L. Pers.) (Chalechale et al., 2015).
The dinitroaniline chemical group has the aniline construction as a basis containing NO2 molecules. Trifluralin and pendimethalin belong to this group with more than ten different herbicides. Trifluralin has been used in agriculture since 1963 (Grover et al., 1997). This herbicide is registered in various countries for controling weeds separately or in mixtures, and it is used in the following crops: Glycine max, citrus, Gossypium hirsutum, Arachis hypogaea, Phaseolus vulgaris, and Allium sativum (Rodrigues & Almeida, 2018). Its application at pre-planting mixed alone with soil or in combination with the post-emergent herbicides is one of the standard methods to control weeds in bean crops (Rouse et al., 2018). Pyroxasulfone is a herbicide that inhibits the biosynthesis of very-long-chain fatty acids (VLCFAs) (Tanetani et al., 2009). This herbicide is a pre-emergent discovered amongst several herbicidal 3-sulfonylisoxa-zoline derivatives (Ito et al., 2015). Another pre-emergent herbicide imazethapyr belongs to the imidazolinone group, a class of herbicides that inhibits acetohydroxyacid synthase in synthesizing branched-chain amino acids in plants (Tan et al., 2005). Imazethapyr is used in weed control of soybeans, alfalfa, corn, rice, and peanuts (Barnett & Brund-age, 2010). The pre-emergent herbicide flumioxazin is an herbicide that blocks protoporphyrinogen oxidase (PPO) activity (Iwashita et al., 2022). Flumioxazin is used in the Fabaceae family since it provides a wide range of protective action against weeds (Norsworthy et al., 2012).
Providing available, effective, low-cost control solutions for the presence of weeds has economic importance in chickpea cultivation. The number of herbicides introduced to control chickpea weeds in Iran and other countries is not comparable to cereal products. Hence, this study was conducted to estimate the appropriate dose and time of application of trifluralin and evaluates the effect of three other herbicides at planting: imazethapyr, pyroxasulfone, and flumioxazin for weed control in chickpea under dryland conditions. Also, possible residual growth effects on wheat growth and yield have been studied.
Materials and methods
The experiment was conducted in the dryland agricultural research sub-institute-Sararood in Kermanshah, Iran (34°20'N, 47°19'E, 1351 m a.s.l.) during the 2018-19 growth season. The climate at the experimental site was semi-arid and moderately cold with long-term total annual rainfall and maximum and minimum rainfall of449 and 171 mm. The average annual temperature was 13.8°C, the absolute minimum temperature was -24°C, and the absolute maximum temperature was 44°C. Total rainfall during the experimental conduction (2018-2019) was 783 mm. Figure 1 shows the monthly precipitation of Sararood station in 2018-2019.
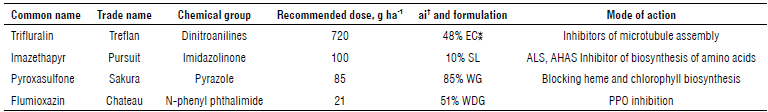
The experiment was performed in a randomized complete block design with four replicates. Treatments applied in 4 x 5 m plot size included trifluralin (48%) applications 30 d before planting (DBP) (480, 720, and 960 g ai ha-1 for treatments 1, 2, and 3); trifluralin applied 15 DBP (480, 720, and 960 g ai ha-1 for treatments 4, 5, and 6); trifluralin applied at planting time (480, 720, and 960 g ai ha-1 for treatments 7, 8, and 9); pyroxasulfone (85%) at planting time (85 ai g ha-1 for treatment 10); flumioxazin (51%) at planting (51 g ai ha-1 for treatment 11); imazethapyr (10%) at planting time (lOOgaiha-1 for treatment 12); weed-infested and weed-free (treatment 13 and 14) (Tab. 1). A Matabi backpack sprayer was used to spray herbicides with calibrated nozzles based on 300 L ha-1 of water. At each stage, immediately after applying the herbicide, a surface disking operation was performed to mix the herbicide with the surface layer of the soil.
Chickpea seeds (cv. Mansour) were planted mechanically using an Aske 2200 (Sazeh Kesht Bukan Company, Iran) on March 19, 2019. Each plot consisted of seven rows with 35 cm row-spacing. The distance between chickpea seeds on planting rows was 8 cm, and the planting depth was 5 cm (35 plants/m2). During two stages, one at the beginning of the growing season and another at the chickpea flowering stage, weeds were manually removed from the plots as weeding check treatment. No control operations were performed in weed-infested (WI) plots. Chickpea harvest was done manually, and seeds and straw were separated and measured manually.
Chickpea growth traits
Measurements were taken at two different chickpea growth stages, in the 8-10 leaf stage (May 8, 2019) and at the beginning of pod formation (May 24, 2019), using a quadrat (with dimensions of 70 x 50 cm) that included two rows of planting with a length of 50 cm. Biologic yield (total biomass + yield), grain yield, plant height, number of pods m2, number of seeds per plant, 100-seed weight, plant dry weight (stems+leaves) at two sampling stages, number of seeds per pod, and plant density of chickpea were measured. In order to measure weed density and dry weight, samples were taken separately from each plot in each treatment. After collecting the samples, weeds were counted per species. Then, to determine the dry weight of the weeds, the samples were dried separately in an oven at 75°C for 48 h.
Wheat traits
These consisted of the visual assessment of the effects of herbicides on wheat growth. The assessment of possible herbicidal effects on the plants was done using a scoring method with a range of 0 to 100. A score of 0 indicated no adverse effect, and a score of 100 indicated plant death. At the end of the growth season, after the complete wheat growth (growth stage 22 according to the Zadox method), the number of tillers was measured in five randomly selected plants in each plot. To measure the 100-seed weight and grain yield, the plot area was harvested and weighed by considering the marginal plot effect at the time of full ripening, and the data were registered in kg ha-1.
Statistical analysis
To determine the richness, the Shannon-Wiener diversity index and their relative frequency at two chickpea growth stages (8-10 leaves) were used using a frame (70 x 50 cm) contained two rows of crop. After collecting the samples, weed plants were counted by species. The samples were then placed in an oven with a temperature of 75°C for 48 h to determine the weed dry weight. Weed species richness, Shannon-Wiener diversity index, and their relative frequency were calculated as follow:
A) Weed species richness indicates the number of weed species present in each treatment (Poggio, 2005); B) Relative frequency of weeds is the ratio of each weed in the sample to the total number of weeds multiplied by 100 (Booth et al., 2003); C) Shannon-Wiener Diversity Index was calculated using the following equations:
where ni is the number of weeds (i) in the sample, and N is the total number of weeds in the sample.
One-way ANOVA procedure was applied using SAS software (Version 8.1) to assess all effects. Significant differences among treatment means were identified by least significant differences test (LSD) (P<0.05) (SAS Institute, 1998).
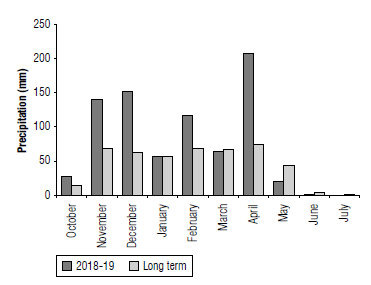
FIGURE 1 Monthly precipitation at Sararood station (Iran) in 2018-19 and comparison with monthly long-term precipitation.
Results and discussion
Weeds
Hare's ear (Bupleurum rotundifolium L.) had the highest relative frequencies in all treatments (average 25%), followed by bitter bean (Sophora alopecuroides L.) (average 11%), field bindweed (Convolvulus arvensis L.) (average 10%), syrian cephalaria (Cephalaria syriaca L.) (average 7%), chicory (Cichorium intybus L.) (average 8%), and prickly lettuce (Lactuca scariola L.) (average 7%). Moreover, other weeds with relatively low frequencies were present in some treatments (Tab. 2). Pyroxasulfone had the lowest relative frequency for hare's ear, although no significant difference was generally observed in relative frequency in different treatments. Researchers stated that the herbicide trifluralin could control lemongrass properly (Mirkamali & Maddah, 1974); also, they reported better control of lemongrass by trifluralin than imazethapyr (Moradi, 2009).
TABLE 2 Relative frequencies of weed species in treatments 30 d after herbicide application. The data is the mean of four replicates.
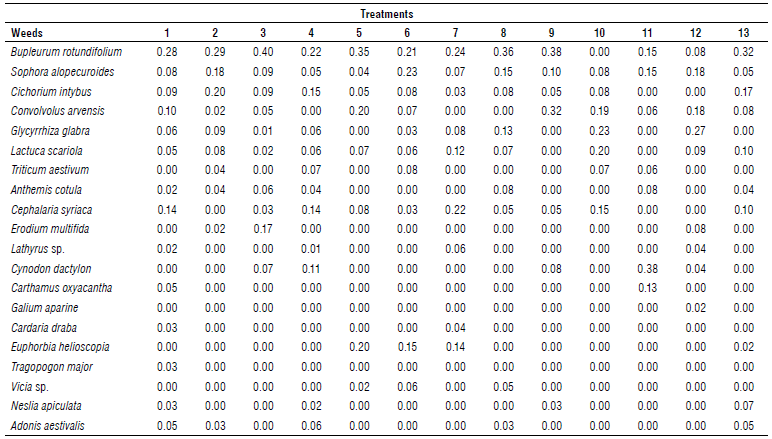
1, 2, and 3: trifluralin 30 d before planting (480, 720, and 960 g ai ha-1), 4, 5, and 6: trifluralin 15 d before planting (480, 720, and 960 g ai ha-1), 7, 8 and 9: trifluralin applied at planting time (480, 720, and 960 g ai ha-1), 10, 11 and 12: pyroxasulfone, flumioxazin, and imazethapyr at planting time, and 13: weed-infested (no weed control).
Results of analysis of variance in the first stage of weed sampling showed that the effect of treatments on the richness of weed species was insignificant (Tab. 3). Most species richness was related to weed-infested plants. On the other hand, treatments 10 and 11 (pyroxasulfone and flumioxazin) had the lowest species richness (Tab. 3). Analysis ofweed species richness variance in the second sampling stage showed a significant difference (P<0.01) between treatments. The herbicide treatment of trifluralin 960 g ai ha-1 at planting had the loweste species richness. The pyroxasulfone and flumioxazin were in the next class, and other treatments were not different from the weed-infested (WI) plot (Tab. 4). Changes in the management of field activities may change the species richness in the field. Field operations may create the conditions for the invasion of one species and make the conditions unfavorable for the presence of other species (Liebman et al., 2001). Managing various factors, especially the chemical management of weeds causes a change in the species richness of the field (Liebman et al., 2001). The combination of various weeds in the field indicates the presence of plants with different abilities in the utilization of water and nutrients that makes it more difficult for the crop to compete with the weeds and then restricts the crop growth (Mousavi et al., 2005).
TABLE 3 ANOVA for weeds richness and Shannon's index.
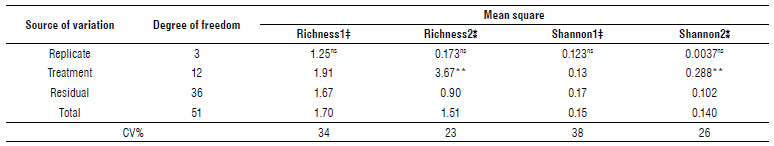
‡ First stage (8-10 leaf stage of chickpea).
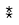 Second stage (the beginning of chickpea pod formation).
Second stage (the beginning of chickpea pod formation).
ns, no significant difference; ** significant difference at P<0.01.
The effect of different treatments on the Shannon diversity index at the first stage of sampling was insignificant, and vice-versa was significant in the second stage of sampling. Flumioxazin and trifluralin at 960 g ai ha-1 had the lowest effects at planting treatments, indicating that these treatments effectively reduced weed diversity. An investigation by examining the diversity index in imazethapyr, trifluralin, and control plots (without herbicide) reported that the value of this index in different stages of chickpea growth in check and imazethapyr was more than trifluralin herbicide treatment (Abbasian, 2011).
Weed density in both sampling stages was affected by herbicides (Tab. 5). In the first stage of sampling, pyroxasulfone, flumioxazin, and trifluralin 960 g ai ha-1 applications at planting produced the lowest number of weeds. These herbicides had 67, 51, and 48% reduction compared to the WI. The other treatments with WI were in the same class. Weed control in two sampling stages has no significant difference in weed density by the chemical control method (Nourbakhsh, 2013). Pyroxasulfone and flumioxazin followed by trifluralin 960 g ai ha-1 at planting resulted in the lowest weed dry weight (Fig. 2) and the lowest weed density (Fig. 3).
TABLE 4 Mean comparison of Richness and Shannon's index in different treatments at two sampling stages.
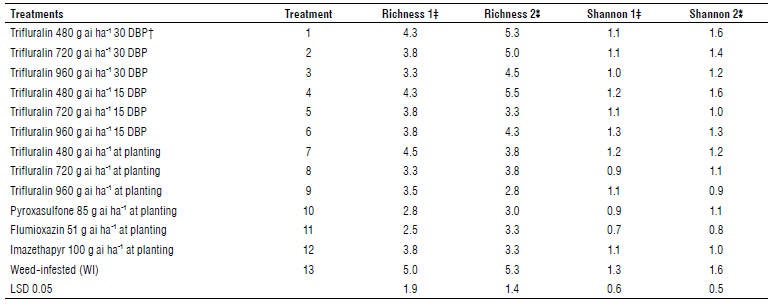
† Days before planting.
‡ First stage (8-10 leaf stage of chickpea).
TABLE 5 ANOVA of weeds density and weeds weight in 2 sampling stages.
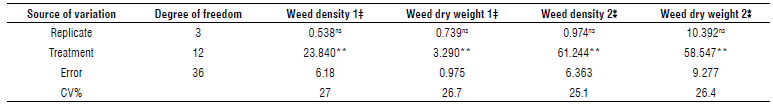
‡ First stage (8-0 leaf stage of chickpea).
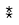 Second stage (the beginning of chickpea pod formation).
Second stage (the beginning of chickpea pod formation).
ns, no significant difference; ** significant difference at P<0.01.
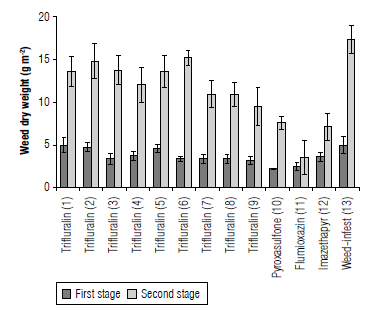
FIGURE 2 Weed dry weight (g nr2) in treatments at two sampling stages (bars represent standard error). 1, 2, and 3: trifluralin 30 d before planting (480, 720, and 960 g ai ha-1), 4, 5, and 6: trifluralin 15 d before planting (480, 720, and 960 g ai ha-1), 7, 8 and 9: trifluralin applied at planting time (480, 720, and 960 g ai ha-1), 10, 11 and 12: pyroxasulfone, flumioxazin, and imazethapyr at planting time, and 13: weed-infested (no weed control).
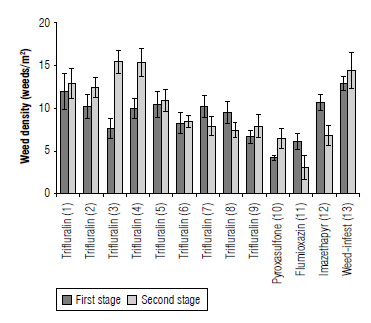
FIGURE 3 Weed density (weeds/m2) intreatments at two sampling stages (bars represent standard error). 1, 2, and 3: trifluralin 30 d before planting (480, 720, and 960 g ai ha-1), 4, 5, and 6: trifluralin 15 d before planting (480, 720, and 960 g ai ha-1), 7, 8 and 9: trifluralin applied at planting (480, 720, and 960 g ai ha-1), 10, 11 and 12: pyroxasulfone, flumioxazin, and imazethapyr at planting time, and 13: weed-infested (no weed control).
TABLE 6 ANOVA for biologic yield (BioY), grain yield (GY), plant height (PH), number of pods per m2 (NSM), number of seeds per plant (NSP), 100-seed weight (100-SW), plant weight at two sampling stages (PW1, PW2), number of seeds per pod (NSpod), plant density (PD) of chickpea in different treatments.
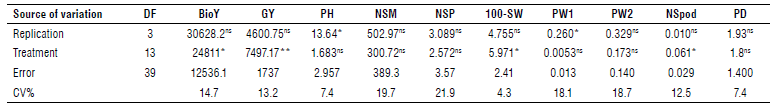
ns, no significant difference; * significant difference at P<0.05. ** significant difference at P<0.01.
Chickpea
The effect of treatments was significant only for the 100-seed weight, biologic yield, and grain yield (Tab. 6). The one hundred seed weight is one of the characteristics related to the quality of chickpea seeds and is essential in terms of marketability and price, since the higher the seed weight, the greater is the chickpea marketability (Abdulahi et al, 2012). The lowest and the highest of 100-seed weight was related to WI and weed-free at 34.2 and 38.2 g. Pyroxasulfone and trifluralin 960 g ai ha-1 at planting were also in this class (Tab. 7). Another study showed that the highest amount of 100-seed weight of chickpea was obtained under weed-free conditions followed by pyridate herbicide, and the lowest 100-seed weight was related to WI (Shahsavari, 2017). When increasing the number of pods and consequently increasing the number of seeds per plant, the 100-seed weight decreased (Samaei et al., 2006). This may be due to the limitations of photosynthetic compounds produced and stored. In this experiment, probably due to drought stress (Tab. 1), the number of seeds per plant was reduced in all treatments, but 100-seed weight of chickpeas was normal and similar to average climatic conditions, and this agrees with the results of others that the sensitivity of this trait to the number of seeds per plant and drought stress is lower (Samaei et al., 2006; Yousefi et al, 2006).
TABLE 7 Mean comparisons of biologic yield (BioY), grain yield, and 100-SW of chickpea in different treatments.
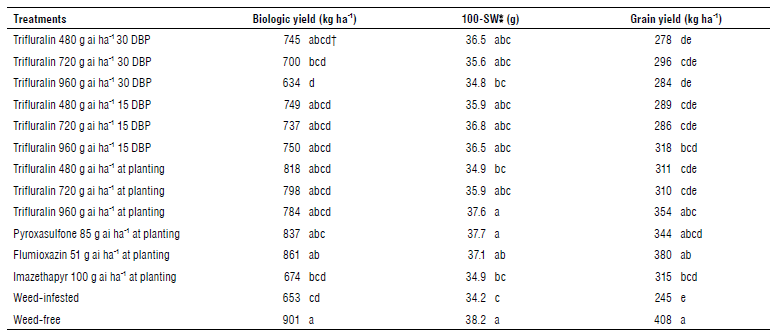
†Means with the same letter in the same column are not signifieantly different according to test LSD (P<0.05).
The highest grain yield was found in the weed-free plots with 408 kg ha-1, and the lowest yield was found in the WI with 245 kg ha-1. The flumioxazin, trifluralin 960 g ai ha-1 at planting, and pyroxasulfone produced 55%, 44%, and 40% higher grain yields than the WI. Many researchers have reported the decreased yield of chickpea in weed competition conditions (Nezami et al., 1997; Mousavi et al., 2007; Nasari, 2010; Abdulahi et al., 2012; Mahmoudi et al., 2012; Nourbakhsh, 2013; Shahsavari, 2017). Another study stated that no herbicide alone can achieve the same grain yield as a weed-free crop (Moradi, 2009); and, therefore, the use of herbicides in this study alone was not sufficient and could not be equivalent to grain yield in a weed-free treatment. Consequently, including a weeding step in the weed management program is necessary.
Wheat growth
Wheat plants in the tillering stage were examined by visual evaluation for residual herbicide effect. None of the herbicide treatments had any adverse effect on wheat growth.
Analysis of variance of wheat tiller number per plant, 1000 grain weight, number of plants per m2, and grain yield of wheat showed that the effect of treatments on these traits was not significant.
Conclusions
Several pre-emergent and pre-planting herbicides have been applied in chickpea crops that helped to control many broadleaf weeds. Even if pre-emergent herbicides control the initial wave of weed growth at the beginning of the growing season, the persistence period of the herbicide may not be able to control the weeds later in the season; late-emerging weeds make it especially difficult to harvest. Therefore, control of broadleaf weeds in chickpea cultivation requires pre-planting herbicides and the subsequent use of post-emergent herbicides or other management methods to control the remaining weeds. Applications of flumioxazin, trifluralin 960 g ai ha-1 at planting, and pyroxasulfone reduced weed number and subsequently resulted in higher grain yields in chickpea. The study of herbicide residual on wheat growth in the next cropping seasons showed no adverse effect.