Introduction
Polyhydroxyalkanoates (PHAs) have been used to replace synthetic petroleum-derived plastics that are environmentally recalcitrant and polluting (Muneer et al., 2020). Advances in biotechnology related to synthetic biology and systems biology can provide efficient strategies to produce PHAs in other organisms, such as genetically modified (GM) plants, by introducing the genes encoding for enzymes involved in the biosynthesis of PHAs in cytosol, plastids, mitochondria, or peroxisomes (Lössl et al., 2003; Anderson et al., 2011; Bohmert-Tatarev et al., 2011). Polyhydroxybutyrate (PHB) is the most common type of PHA (Stouten et al., 2019), which is synthesized from acetyl-CoA by the bacterium Cupriavidus necator involving three enzymes: (i) (3-ketothiolase (phaA gene) catalyzes the condensation of two molecules of acetyl-CoA to acetoacetyl-CoA; (ii) acetoacetyl-CoA reductase (phaB gene) catalyzes the reduction of acetocatyl-CoA to (R)-3-hydroxybutyryl-CoA; (iii) PHA synthase (phaC gene) catalyzes the polymerization of (R)-3-hydroxybutyryl-CoA monomers (Gupta et al., 2021).
Acetyl CoA is produced in numerous metabolic pathways in plants. In general, the metabolic pathway of glycolysis occurs in the cytosol; once pyruvate has been produced it is oxidized by pyruvate dehydrogenase into acetyl-CoA to enter the citric acid cycle. The acetyl-CoA can be used by the β-ketothiolase present in the cytosol to carry out the first metabolic reaction that produces PHB. Insertion of phaB and phaC genes into the plant genome is required to complete the synthesis of PHB in the cytosol (Jeon et al., 2014). The production of acetyl-CoA in plastids is obtained through de novo biosynthesis of fatty acids, and the synthesis of PHB in this organelle can be carried out through the insertion of the three PHA related bacterial genes (Bohmert-Tatarev et al., 2011). Plant peroxisomes produce 3-hydroxyacyl-CoA intermediates through β -oxidation of fatty acids that can be used as precursors in the synthesis of PHAs; therefore, the biosynthesis of peroxisomal PHB can be obtained by inserting a phaC gene into the nuclear genome and targeting the enzyme to peroxisomes with a transit peptide (Arai et al., 2002; Tilbrook et al., 2014). PHA synthase from C. necator produces PHB from acetyl-CoA in the cytosol and plastids, while PHA synthases from Aeromonas caviae (phaC A.c gene) and Pseudomonas aeruginosa (phaC1 gene) produce PHB (cytosol, plastids and peroxisomes) and different PHAs using 3-hydroxyacyl-CoA intermediates (Jeon et al., 2014; Jia et al., 2016).
The development of commercial PHA-producing GM plant lines must take into consideration elements such as transformation vectors, genes, regulatory regions, proteins, strains, plant genotypes, and plant transformation protocols. These elements may be protected by intellectual property rights (IPR): patents, plant variety certificates, confidentiality agreements, and material transfer agreements (Carroll, 2016). However, legal aspects related to patents can circumvent IPR and prompt the development of PHA-producing GM plants. Patents expire due to nonpayment of maintenance or the completion of the protection period, leaving the invention in the public domain where the authorization of its original owners is no longer required for its use. Likewise, patents have national jurisdiction, meaning they are only valid in the countries where they were requested and granted (Wolf, 2008; Heikkilá & Lorenz, 2018).
The development of a GM plant line for commercial purposes requires a related IPR analysis to verify if the elements involved are protected and, if so, the term and the countries in which its development, application, and commercialization do not infringe the rights of third parties. The number of patents protecting this technology can increase the costs associated with development due to the payment of royalties or licenses (Alandete-Saez et al., 2015).
Nicotiana tabacum is one of the most used plants for genetic transformation. Its plasticity, susceptibility to transformation mediated by Agrobacterium tumefaciens, and ability to generate biomass places GM tobacco as a potential green factory for the production of proteins for medical or industrial use (Buyel & Fisher, 2012; Ibrahim et al., 2019; McNulty et al., 2021; Fearon et al., 2022).
This study analyzes the IPRs status through patents on PHA-producing GM plants worldwide, focusing on the design of a GM tobacco line that expresses the phaC Ax gene from A. caviae for the synthesis of PHAs in peroxisomes in order to identify patent-free elements for the development of a GM tobacco event producing agrobiogeneric PHB.
Materials and methods
A search in public access patent databases was performed in order to identify: (i) patents that protect the development of GM PHA-producing plants in the world, and (ii) patents that protect elements used in the design of a GM tobacco line that expresses the phaC A c gene. The Lens (https://www.lens.org), Espacenet (https://worldwide.es-pacenet.com/), and Google Patent (https://patents.google.com/) databases were used. In addition, patents requested through the Patent Cooperation Treaty (PCT), directed by the World Intellectual Property Organization (WIPO), were also considered.
PCT allows the applicants to simultaneously request protection of their invention in signatory countries with a single application. Applicants have 30 months to request a patent in the desired regional office (Barreto et al., 2020). The search terms used to identify the appropriate Classification Symbols of the international patent code (IPC) were: "Genetic term", "Genetic engineering", "Mutants or genetically engineered organisms", "Mutations or genetic engineering" and "New plants per se". Patent priority dates, current maintenance status, application through the PCT, and jurisdictions were established. An analysis of the trend of patent requests over time was performed.
The patent status of the elements used in the development of the GM line that expresses the phaC A.c gene was done with the IPC terms mentioned above and the following keywords: "phaA gene", "phaB gene", " phaC A c gene", "PHAC synthase from Aeromonas caviae", "CaMV35S promoter", "CaMV35 promoter enhancer", "T-Nos terminator", "Target in malate synthase signal", "Transgenic producer of polyhydroxyalkanoates", "Production of polyhydroxy-alkanoates in tobacco", "Polyhydroxyalkanoates", and "PHA Copolymer". The collected patent application data consisted of patent application families (patent documents requested in several countries that protect the same invention). It included their legal status, priority date, geographic coverage, and claims. The search was conducted from April 2019 until December 2021. All data were processed with R software.
Results and discussion
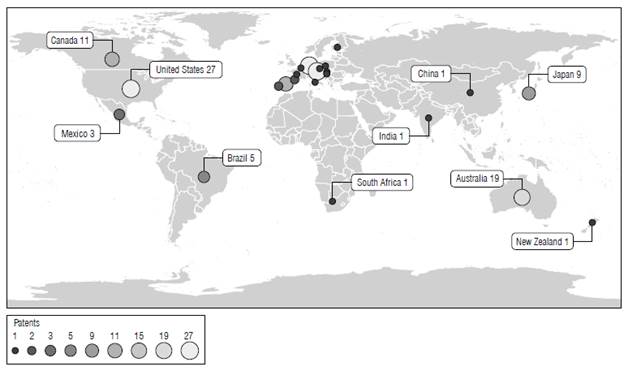
Patent families and patent applications related to the development of PHA-producing GM plants were explored in worldwide databases (from 1991 until 2010). Twenty-three patent families were assigned through PCT, and six patents requested at a national patent office were identified. The United States of America (US) has the highest number of applications, followed by Australia and some countries of the European Union (EU) (Fig. 1).
Eighteen patent families expired from 2012 to 2020. Patents in these families were granted in the US, Australia, the EU, Brazil, Mexico, Japan, India, and China. Eight patent families expired between 2004 and 2018 because annual maintenance payment was not recorded. Four patent families remain valid and will expire between 2022 and 2030; these patents were granted through the PCT in the US, Brazil, and Australia. GM seeds covered by patents limit the use of this technology because profitability is generated for crops cultivated in large areas.
PHA synthesis in the cytosol, plastids, and peroxisomes of GM plants has been generated from intermediary substrates of metabolic pathways such as glycolysis, fatty acid synthesis, and fatty acid degradation. The identified patents protect the development of GM plants that produce PHAs in the cytosol, plastids, and peroxisomes. Strategies to improve PHA synthesis in these subcellular compartments are WO1992019747, WO1993002187, WO1994012014, WO2004006657, US8487159, and WO2011034945. These protect GM plants such as Arabidopsis thaliana, cotton, switchgrass, sugarcane, and oil crops, which produce between 1.6% and 7% of PHB per unit of dry cell weight in the cytosol by insertion of phaA,phaB, and phaC genes from C. necator, Pseudomonas oleovorans, and Zoogloea ramigera, respectively. Patent application WO2012037324 covers the development of GM turf and poplar in which the carbon flux in the Calvin-Benson cycle was increased by the insertion of genes coding for sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphosphatase, transketolase, or aldolase to improve PHB production (Tab. 1).
Patent applications WO1990061624 and US6228623 cover a method to produce polyhydroxypropionate, PHB, poly(3-hydroxypropionate-co-5-hydroxyvalerate) copolymers poly(3-hydroxypropionate-co-3-hydroxyvalerate), poly(3-hydroxybutyrate-co-4-hydroxyvalerate), poly(4-hydroxybutyrate-co-3-hydroxyhexanoate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate-co-3-hexanoate) in the cytosol of GM plants and in bacteria (Tab. 1).
Patent application WO1999000505 protects a genomic fragment and a method to enhance levels of substrates needed to generate the copolymers 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV) through the insertion of phaA, phaB, phaC genes and encoding enzymes involved in the biosynthesis of the aspartate family amino acids and threonine deaminase.
Patent applications WO199505472, WO2010102217, and US6620601, protect a methodology to produce up to 10% PHB per unit of dry cell weight in A. thaliana and N. tabacum plastids by insertion of phaA, phaB, and phaC genes fused to a transit peptide to direct polymer biosynthesis in plastids. Granted patent WO200202040690 describes a GM plant expressing transgenes encoding the enzymes 3-hydroxyacyl ACP thioesterase and PHA synthase to produce PHA from fatty acids in plant plastids. Granted patent WO1992019747 describes a GM Brassica plant that produces PHAs when the genome contains genes encoding enzymes for the PHA biosynthetic pathway in the cytosol or plastids (Tab. 1).
Patent applications WO2001023596, WO1999035278, WO2000043523, and WO2010057271 cover a method to produce 1-2% dry weight of PHA polymers and co-polymers in A. thaliana and sugarcane peroxisomes through the insertion of genes encoding 3-ketoacyl-CoA reductase or 2-enoyl-CoA hydratase and acetyl trans-ferase attached to a transit peptide to direct peroxisome biosynthesis. These enzymes are derived from A. caviae, C. necator, P. putida, and Klebsiella aerogenes (Tab. 1). Patent WO1994011519 protects a PHB production system in oil seed crops transformed with a gene that mediates PHA biosynthesis fused to a switch gene induced by a chemical substrate that allows the synthesis of PHB in the cytosol or glyoxysomes of the seeds. Patent applications WO1998006854 and WO1999045122 request the protection of a methodology to modify the biosynthesis of fatty acids and produce polymers in plants by insertion of genes encoding the enoyl-CoA hydratase and epimerase from P. putida FaoAB involved in PHA biosynthesis, directing the enzymes towards the peroxisomes or plastids.
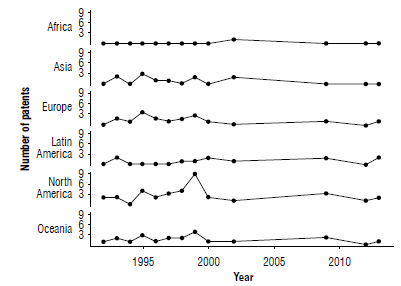
A patent family analysis on the production of PHAs in GM plants was performed to identify if these documents indicate the countries where the applicants seek technology protection. Demand for patents on PHA production in GM plants is highest in countries of North America followed by countries from Oceania (Fig. 2).
Trends indicate an average of 1.52 patent applications per year; the highest demand for patent applications was in 1999 in North America, Oceania, Europe, and Asia, while in Latin America the highest number of patents was registered in 1998 and 2009 (Fig. 2). This information shows a transition of patent demand since 1992, the year in which the first patent applications were registered. The number of patents related to this technology grew from 1995 to 1999; no applications were found between 2003 and 2007. The period from 2000 to 2010 shows a decrease in the rate of new advances. These data reveal that this technology could be classified as emerging in Latin America and as declining in North America, Oceania, and Europe.
The reduction in patent requests since 2000 maybe because the technology still has not obtained enough yields in the production of biopolymers, and efforts have focused on microbial fermentation as demonstrated by Kosseva and Rusbandi (2018) improving the availability of substrates with a high carbon source and optimal growth conditions. Dobrogojski et al. (2018) suggest that it is necessary to increase the efficiency in the accumulation of PHAs in plants by optimizing the tissue conditions where PHAs are stored.
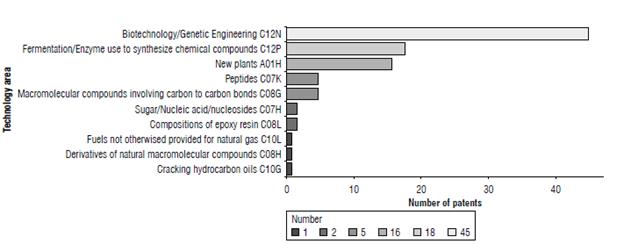
The technological areas related to PHA-producing GM plants patents are clustered in the groups: "human needs" and "chemicals and metallurgy" assigned by the IPC with A and C codes, respectively. The subclasses are plant reproduction by tissue culture techniques (A01H), biotechnology and genetic engineering (C12N), fermentation or enzymes using processes to synthesize a desired chemical compound (C12P), cracking hydrocarbon oils, production of liquid hydrocarbon mixtures (C10G), fuels not otherwise provided for (C10L), macromolecular compounds obtained from reactions not involving unsaturated carbon to carbon bonds (C08G) peptides (C07K) and sugar/nucleic acid/ nucleosides (C07H). Applications across the IPC database is concentrated in the areas of biotechnology and genetic engineering (88.23%) followed by fermentation (35.29%), new plants (33.33%), and, in lesser percentage, peptides (9.8%), etc. (Fig. 3). Patent search in the IPC database not only facilitated the recovery of documents in the previously mentioned areas, but documents in other less common areas such as the composition of the epoxy resin or cracking hydrocarbon oils were also retrieved. Given that the IPC identifies each of the components that make up the technology, this system is useful for patent examiners and inventors to find documents that help identify the state of the technique.
Forward citation analyses are relevant to determine the progress of scientific knowledge related to the patented activity and to identify the relationship between science and technology for industrial development (Verbeek et al., 2003). An analysis of citations on the identification of PHA-producing GM plants patents across 1992-2013 found that old patents are cited more than recent ones, from 1992 to 1993. WO1994012014 was cited 45 times; between 1994 to 1995, WO199 2019747 was mentioned 67 times; between 1996 and 1997, US6228623 was cited 88 times; between 2009 and 2010, WO2010102217 was mentioned 29 times; from 2012 to 2013, patent WO2012037324 was cited three times, and patent WO2011034945 was cited twice. The number of forward citations is an indicator of technological, social, and economic value of patents (Harhoff et al., 2002). In this case, patents such as WO199 2019747 and US6103956 represent a benchmark of innovation. Patent WO1992019747 was requested by the company Metabolix Inc. in the US, Australia, United Kingdom, and EU and had its highest peak of citations between 1998 and 2001. Patent US6228623 was requested by Metabolix Inc. and Bayer/Monsanto; its highest peak of citations was between 2010 and 2013. On the other hand, the low number of citations of recent patents, such as WO2012037324 and WO2011034945, is, probably, because, as of 2013, the technology has not shown a major advance in new applications (Tab. 1).
A freedom to operate (FTO) analysis was carried out for the development of GM tobacco events expressing phaC gene from A. caviae with the lowest possible patent load. Patent claims identification was done for the relevant elements involved in the making of a GM tobacco with the desired trait. It identified elements that composed this biotechnological product (vectors, transformation methods or genes) and analyzed the claims of the patents. Similarly, countries where requests and grants of a patent were made were determined as well as the patent status (Nagori & Mathur, 2009; Hincapié Rojas & Chaparro-Giraldo, 2014). These reports show limitations in the claims of the patents which may favor the development of new free patent products or facilitate the negotiation of licenses with the owners. Relevant patents were divided into five sections: A. tumefaciens strain and transformation method, promoters and transcription terminator, cloning vector, phaC AC gene, and transit peptide.
A tissue culture protocol and a genetic transformation method must be established initially to obtain a GM tobacco line. Transformation mediated by A. tumefaciens strain LBA4404 is accomplished using leaf fragments as explants that generate new seedlings by direct organogenesis. Four patent families protect A. tumefaciens strain LBA440 as a method to transform dicotyledonous and monocotyledonous plants in a stable or transitory manner. Patent WO2004082368 protects this strain as an optimized method of genetic transformation based on A. tumefaciens-mediated transformation of Brassica júncea and was granted in Australia, the US and the EU; this patent will expire in 2023. Patent WO2013149726 protects a transient leaf transformation process using strain LBA4404; it was registered in Brazil and the US and was granted in Argentina, Australia, Canada, China, Mexico, Japan, and the EU. This patent will expire in 2032. Patent WO2010078445 covers a method of plant cell transformation and regeneration mediated by auxotrophic strains of A. tumefaciens (LBA4404, EHA101, C58, EHA105, AGL1, or GV3101) carrying a mutation in the thyA gene, a condition that prevents their growth in culture media lacking thymidine. This patent was requested in Australia and Canada and was granted in the US and China. Although it is valid until 2028, according to the information retrieved in the Lens database, no maintenance payment has been recorded since 2016. Therefore, it expired in February 2017. Patent WO2012016222 covers A. tumefaciens strains with genes that enhance transformation and are located in plasmids capable of replicating independently of the Agrobacterium chromosome. This patent was requested in Colombia, South Africa and Brazil and was granted in Australia, China, the EU, Mexico, the US, and Russia; it is valid until 2030. Patent W02010078445 is related to the transformation method, covering a process to produce a GM plant through systemic infection with an auxotrophic strain of A. tumefaciens LBA4404. This patent expired in 2017. Patent WO2004038023 covers an improved method of plant transformation with A. tumefaciens through a time interval between the preparation, inoculation, and co-culture stage. This patent was requested in Mexico, the EU, Canada and was granted in Australia, the US and Russia; it is valid until 2022. Patent application WO2012098119 was granted to the Philip Morris Company in Brazil, China, Japan, the EU, the US, and Russia. This patent protects a transformation method to transiently express recombinant proteins through pressure infiltration of empty leaves of N. tabacum with A. tumefaciens. This patent will expire in 2031.
For the transcription of the phaC gene inserted into the plant genome, a 35S promoter from the cauliflower mosaic virus (35S CaMV) and the nopaline synthase terminator from A. tumefaciens (T-nos) is used. According to the information retrieved from the databases, one patent family protecting the CaMV 35S promoter was identified. Granted patent US5352605A protects the 35S CaMV promoter and the 19S CaMV promoter as part of expression constructs and plant transformation vectors in dicotyledonous plants using chimeric constructs. These patents expired in 2003; therefore, this promoter is already in the public domain. Likewise, there is a version with a duplicated 35S CaMV promoter that has an enhanced overexpression of the gene. Patent application US5164316 protects a transcription start sequence formed by tandem sequences of the 35S CaMV promoter and covers its use in plant genetic transformation. This patent expired in 2007. The terminator sequence of the T-nos expression construct is associated with patent US5034322, which protects chimeric genes containing the nopaline synthase promoter (A. tumefaciens) and a 3'-un-translated region of the nopaline synthase gene as well as the terminator sequence. This patent expired in 2003.
A group of vectors suitable for plant transformation is pCAMBIA plasmids. The Center for the Application of Molecular Biology to International Agriculture (CAMBIA) is a nonprofit research center in the field of botanical biotechnology that focuses on reforming the open science innovation system and intellectual property. pCAMBIA vectors are binary because they have an origin of replication in Escherichia coli and A. tumefaciens and contain the T-DNA region that houses the gene of interest to be transferred to the plant. CAMBIA has generated an open-source license in which the use of these vectors does not generate a royalty payment for institutions with research, academic, or nonprofit purposes, as well as for developing countries (CAMBIA, 2022). The expression constructs containing the coding region of the region of the phaC A.C gene from A. caviae assembled with the 35S CaMV promoter and the T-nos terminator are assembled into a vector of the family pCAMBIA; therefore, patents associated with pCAMBIA vectors were searched. Patent US20090075358 was granted in the US. Under this patent, two families of vectors are protected; the first covers vectors containing at least one functional origin of replication for non-rhizobial species and a T-DNA border sequence linked to the sequence of interest; the second family covers the vectors that contain a functional origin of replication in at least one non-rhizobial species and harbor virA, virG, virJ operons, and a mutant virG. This vector is incorporated into a bacterial host chromosome. This patent protects their use in the transformation of plants by A. tumefaciens. The patent expires in 2027 (Tab.1).
The phaC Ac is associated with five patent families and one patent application; patent application WO200011188 covers recombinant strains of microorganisms with the genes involved in PHA biosynthesis, where the gene encoding for PHA synthase was derived from A. caviae. This patent expired in 2018 (Tab.1). Patent WO2000043523 protects a method to produce PHA-producing GM organisms through the insertion of a PHA synthase gene from A. caviae, Co-mamonas testosteroni, and Thiocapsa pfennigii, among others. This patent was granted in Australia, Canada, the EU, and Japan. The payment of the maintenance fee was not made in 2016, according to the search done in the Lens database, and it lost its validity on January 17, 2017 (Tab.1). Patent WO2011060048, accepted through the PCT, protects a recombinant C. necator, which harbors the phaC A c gene from A. caviae to produce poly(hydroxybutyrate-co-hydroxyhexanoate) copolymer; this patent is valid until 2033 in China, Japan, South Korea, Australia, the US, and the EU (Tab.1). Patent WO2015146195, granted in the EU, the US, Australia, and Japan, covers a method for the improvement of PHA copolymer synthesis using a microorganism with a gene encoding for a PHA synthase of Aeromonas sp. and a gene encoding for a different PHA synthase to produce two or more PHAs with different melting points; this patent will expire in 2035 (Tab.1). Patent US7384766, granted in the US, protects a transgenic microbial strain which contains a gene encoding PHA synthase from A. caviae to produce a PHA copolymer constituted by monomer units from 3-hydroxybutyric acid, 3-hydroxyhexanoic acid, 3-hy-droxyheptanoic acid; this patent is valid until 2028 (Tab.1).
Studies conducted to produce biopolymers in tobacco, directing their biosynthesis towards chloroplasts at the nuclear level, have produced plants with chlorosis and delayed development (Lössl et al., 2003; Matsumoto et al., 2011). In other species, such as Arabidopsis and sugarcane, where the synthesis of biopolymers is targeted towards peroxisomes, no deleterious effects were observed in the plants (Matsumoto et al., 2006; Anderson et al., 2011; Tilbrook et al., 2011). An Ala-Arg-Leu (ARL) transit peptide is attached at the C-terminal end of the expression construct containing the coding sequence of the phaC A.c gene from A. caviae to direct the enzyme PHA synthase towards peroxisomes (Tilbrook et al., 2014) (Tab.1). The transit peptide ARL is associated with three patent families assigned in Australia, Canada, Japan, the EU, and the US (WO1999035278, WO2000043523, and WO2010057271). These patents cover the expression of a PHA synthase and its targeting to plant peroxisomes by tripeptides Ala-Arg-Met, Ser-Arg-Met, Ser-Lys-Leu, Ala-Arg-Leu, Ser-Arg-Leu, Pro-Ser-Ile or Pro-Arg-Met, and the production of PHB in sugarcane peroxisomes by targeting phaA, phaB, and phaC gene products using a Arg-Ala-Val-Ala-Arg-Leu (RAVARL) signal sequence or any similar functional fragment. Patent WO1999035278 lost its validity in 2018, while patents WO2000043523 and WO2010057271 are valid until 2023 and 2028, respectively (Tab.1).
Plant breeder's rights (PBR) is a sui generis IPR resource used to protect varieties developed by genetic improvement, and the person who obtains these new varieties is called a breeder (UPOV, 2022). The granting of PBR entitles the holder to ownership of that variety and any person wishing to commercialize it must be authorized by the holder (breeder). This authorization is generally granted in the form of a license agreement. A variety can be protected by PBR if it meets all the following criteria: novelty, distinction, uniformity, and stability; it must also be given an appropriate denomination. The PBR is valid for 25 years from the grant date in the case of fruit trees, forest trees and vines and for 20 years in other species. Its validity is jurisdictional and is in force in the country where it was granted; however, if it is requested through intergovernmental organizations, the right is valid in all member states of the organization (ICA, 2017). The intergovernmental organization, the International Union for the Protection of New Varieties of Plants (UPOV), promotes an effective system of plant variety protection; its database (Pluto) contains plant breeder's titles requested in UPOV member countries for different plant species. UPOV members are eligible for the breeder's exemption, which allows all breeders to use protected varieties for further breeding activities (UPOV, 2013). PBR registrations for N. tabacum were identified in the Pluto database in the US, Canada, Mexico, Argentina, Colombia, the EU, Russia, South Korea, and China. The US and Bulgaria were the countries with the most PBR registrations for this species from 1950 to 2022. Ninety-six varieties were identified; protection is still in force for 22 of them. Some of the varieties that are in force are: KF109 (South Korea), KB111LC (South Korea), Wan6513 (China), Guang Yang (China), Vector 21-41 (Canada), Speight 22 (US), AOB176 (US), MB47 (Argentina), and MB411 (Argentina).
Once the patents expire, it is possible to develop generic seeds with the technology without prior authorization from the owner (Jefferson et al., 2015). The use of the technology without the need to pay for a license or royalties reduces costs of production and development and, increases access to technology for small-scale farmers or developing countries.
Where an agbiogeneric crop is generated, the developer of that GM crop must face the challenges of opening up a commercial authorization, especially those related to discordant harmonization between procedures because the regulations issued by the countries vary widely, not only in quantity but also in the level of aspect (Jefferson et al., 2015). GM crop regulation varies depending on whether the events belong to first, second or third generation. First GM generation events have traits such as herbicide tolerance and insect resistance, second GM generation crops have traits related to food and feed quality while third generation GM events comprise recombinant proteins or industrial products (e.g., pharmaceutics or biodegradable polymers) (Spök et al., 2008). The Cartagena Protocol on Biosafety (Law 740 of 2002 in Colombia) contains specific regulations on GM organisms. In essence, it is a normative body that dictates policies and suggested actions for the different dimensions of intended use, whether these are to be exported or imported or cultivated for food or feed (Turnbull et al., 2021). First and second generation GM crops projected for feed usually require substantial equivalence studies, a comparison of a GM product and its conventional counterpart, including an agronomic performance assessment and tests to determine any adverse effect on environment or human health (allergenicity and toxicity) (McHughen, 2016; National Academies of Sciences Engineering and Medicine, 2016). For third generation GM events, in addition to agronomic, environmental, and human health biosafety requirements, an environmental commercial release plan must be provided to ensure that there will be no contamination of seeds used for human or animal consumption (Spök et al., 2008).
In some countries, regulations are centered around GM crops for food and animal feed, while in other countries, the focus is more on importation of GM products for human consumption. In the US, the GM crops are regulated by different agencies (USDA APHIS, 2022). In contrast, Canadian regulations for GM crops are based on novel traits, so they may also be described as having a new trait rather than as a GM crop in itself, with methods not taken into account (CFIA, 2020). The Council of the European Union passes regulations, usually focusing on the GM products imported to their own countries rather than cultivation (Eriksson et al., 2020). In the case of Latin America, there are efforts to achieve a harmonized regulatory framework by crops and products, with a case-by case examination for those countries where use and cultivation is allowed, including Brazil, Argentina, Colombia, Costa Rica, Honduras, Paraguay, and Uruguay (Benitez et al., 2020).
A global data package containing the tests and evaluations performed by the holder of the expired patent is a relevant step in a series of conditions designed for the commercial approval of an agbiogeneric by the authorities in the countries where GM events can be marketed for cultivation (Rüdelsheim et al., 2018). When a GM event is deregulated in the US, it can be freely marketed without the need for new approval requests unless new traits are introduced or there are changes to the GM event (USDA-APHIS). In the European Union, China and South Korea among others, authorizations for an approval extension must be requested by providing updated information and periodical reports (Rüdelsheim et al., 2018). If a GM event is no longer protected and the original inventor or patent holder no longer has an updated approval for a specific country, an agbiogeneric cannot be commercialized in that country, given that the original developer holds the rights to the regulatory data and the regulatory offices considers them confidential. An alternative would be for the agbiogeneric developer to reach an agreement involving a license (Rüdelsheim et al., 2018). The other option is to initiate a regulatory process with own data by tests specific to regions where the commercial approval is planned. It is also important to note that if an agbiogeneric represents a new GM event, the developer must provide information about insertion location and effects on agronomic traits. A common cost-cutting measure is to negotiate management agreements to access and use general information valid for several events (transgene source, nucleotide sequence, protein, genetic trait, allergenicity, toxicity) (Rüdelsheim et al., 2018).
Conclusions
The patent analysis related to PHA-producing GM plants shows that, for some countries (e.g., the US or Australia), development has not continued with the same intensity. This technology in Latin America is just launching. Of the six patent applications for PHA-producing GM plants, only four are in force: WO2004006657 expires in 2022, WO2010102217 and WO2010057271 expires in 2029, and WO2012037324 expires in 2032. Upon expiration, the protected invention becomes public; a generic manufacturer can develop the PHA-producing GM tobacco agbiogeneric, such as drought-tolerant GM corn or glyphosate-tolerant GM soybean (Zanga et al., 2015; Rojas Arias et al., 2017). Up to the date of this study, there is no patent that includes PHA-producing GM tobacco using the A. caviae phaC gene for in the peroxisome synthesis.