Introduction
Phyla nodiflora var. minor (synonym P. canescens (Kunth) Greene) (Verbenaceae), commonly known as "lippia" in Australia, is a notorious weed of riparian and floodplain production and conservation areas with considerable impact on rural production, land values, and ecosystem services. It is a perennial plant with a prostrate habit and creeping stems that can root at each node, which favors spreading and increasing in density. This invasive plant was commercially introduced in Australia as an ornamental species during the second half of the XIX century and has invaded 5.3 million ha of the "Murray-Darling" basin floodplain. The greatest invasions and impacts occur in the Murray-Darling Basin's northern catchment, but also throughout the basin and elsewhere in Australia. In the Murray-Darling Basin, the weed costs the grazing industry an estimated Australian dollar AUD $38 million per year and the environment AUD $1.8 billion per year (Earl, 2003).
Current short term and unsustainable control methods include the use of herbicides, cultivation, and grazing management (Julien et al., 2012). The use of herbicides is restricted by the presence of susceptible crops along waterways. Phyla nodiflora var. minor may be managed by cultivation, however, in many areas the practice is not sustainable as there is a significant risk of soil loss associated with cultivation of areas adjacent to waterways (Earl, 2003). Biological control was proposed as part of the weed management in reserve areas, woodlands, forests, and along stream banks.
Originally, two species were recognized in Australia, Phyla nodiflora and Phyla canescens (Munir, 1993). Although Phyla nodiflora has a worldwide distribution, the condition of being native to Australia (Gross et al., 2017) has implications for the selection of biological control agents. The South American genus Phyla Lour (Verbenaceae), originally included in the genus Lippia L., currently comprises five species (O'Leary & Múlgura, 2012). Following the taxonomic revision of the genus Phyla Lour provided by O'Leary & Múlgura (2012), three varieties of P. nodiflora are recognized: P. nodiflora (L.) Greene var. nodiflora, P. nodiflora var. minor (Hook.) O'Leary & Múlgura, and P. nodiflora var. reptans (Kunth) Moldenke. According to Sosa et al. (2017), the variety minor is an invasive weed in Australia, while the variety nodiflora is the native Australian form (formerly known as P. nodiflora).
The P. nodiflora plant is native to South America with the center of origin probably in Central and Northern Argentina (Julien et al., 2012). It is likely that many specific antagonists can be found in this region, and they can be proposed as potential biological control agents against this weed. Systematic surveys on the three varieties of P. nodiflora are scarce, and only a few arthropods (Cabrera et al., 2016) and pathogens are mentioned as natural enemies (Viégas, 1961; Ellis, 1976; Farr et al, 1989).
Puccinia lantanae Farl. (Pucciniales, Basidiomycota) was described as infecting P. nodiflora var. minor (formerly Lippia canescens=P. canescens) in Argentina (Lindquist, 1982). Julien et al. (2012) registered this rust damaging P. nodiflora under natural conditions in the field in Argentina. Puccinia lantanae is an autoecius (completes the life cycle on a single host) microcyclic rust, for which only the teleutosporic stage is known (Cummins & Hiratsuka, 2003). This rust has been reported on Lippia spp. in South America ( fideJackson, 1932; Lindquist, 1982). Particularly, in Argentina, the geographic distribution range of P. lantanae in Lantana spp. and Lippia spp. includes Buenos Aires, Entre Ríos, Corrientes, Misiones, Tucumán and Salta provinces (Hernández & Hennen, 2002). Previous studies suggest its potential use as a biocontrol agent of Lantana camara L., considered a weed with serious impact (Barreto et al., 1995).
Rusts are considered very attractive biocontrol agents due to their high specificity and the impressive biocontrol successes in some species (Morin et al., 2006), with different pathotypes specialized on different plant species and even in some cases on different genotypes within the same plant species. Additionally, there is evidence of distinct races of P. lantanae that attack only one species and are even specific to biotypes within that species (Rentería & Ellison, 2004).
Field surveys in the native range and knowledge of the biology of putative biological agents are essential components of a biocontrol program (Fourie & Wood, 2018). Extensive collecting and research on this rustwere performed in Brazil and Peru (Barreto et al., 1995; Ellison & Cortat, 2011), but similar studies do not exist for Argentina or bordering countries. Temperature is one of the environmental limitations of the disease development in the field (Agrios, 2005); its effect on teliospore germination and basidiospore production is crucial in the epidemiology of microcyclic rust disease.
In this research, we aim to study: i) the geographic distribution of P. lantanae on P. nodiflora in Argentina and two bordering countries (Bolivia and Chile), (ii) the effect of different incubation temperatures, aging and heat shock on teliospore germination and basidiospore formation, and (iii) the pathogenicity of the rust fungus on P. nodiflora var. minor, P. nodiflora var. reptans, and P. nodiflora var. nodiflora.
Materials and methods
Field surveys and collection of rust isolates
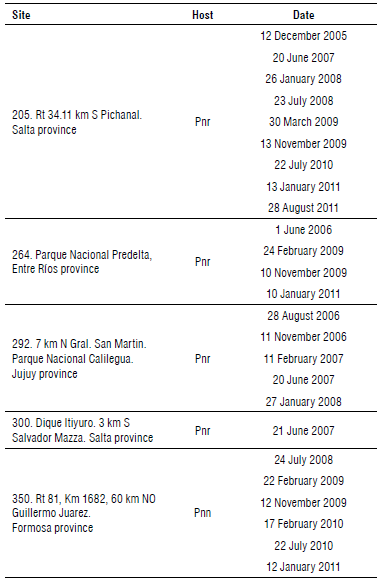
Field trips were performed to study the geographic distribution of Puccinia lantanae in Argentina, Bolivia, and Chile. Both rust and P. nodiflora (var. minor, reptans, and nodiflora) plant material was collected from different locations. Roadside surveys were conducted in Argentina from December 2004 to January 2011 (41°S northwards). As P. nodiflora var. minor is a small and prostrate plant and often grows in the understory, it is difficult to observe from a moving vehicle. Therefore, inspection sites were assigned according to the following criteria: the first site was randomly selected within the first 50 km away from Buenos Aires along main routes and subsequent inspections were systematically placed every 50, 100 and 180 km, giving a total of 217 visited sites during 6 years of field survey (Fig. 1). Plant specimens from all locations were collected and dried for further identification. Whenever a rust infected plant population was found, material was collected and placed between pieces of newspaper in a plant press for further study in the laboratory (Tab. 1). Each collection of the rust is termed "isolate". A GPS reading was taken to record the site location. Some sites were visited more than once in different seasons.
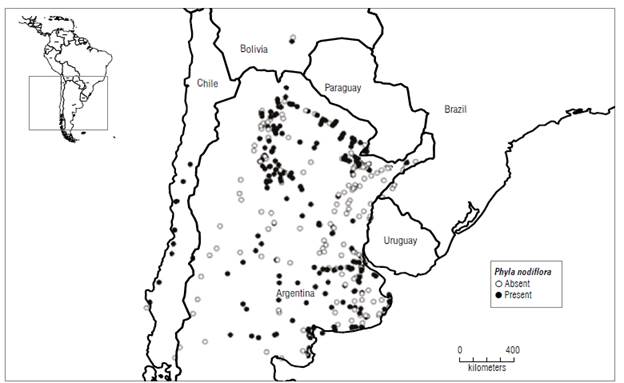
FIGURE 1 Distribution of Phyla nodiflora var. minor, reptans, and nodiflora in southern South America. Circles indicated sites visited to sample the plants. Solid circles: plant present, empty circles: plant absent. Data from Chile were obtained by A. Sosa.
TABLE 1 Rust collection sites and dates. Pnr: Phyla nodiflora var. reptans. Pnn: Phyla nodiflora var. nodiflora.
In the laboratory, field collected samples of infected plants with rust were processed. Freehand razor blade cuts were performed on infected leaves. The cuts were placed in water or potassium hydroxide in an aqueous solution 5%. A coverslip was placed over the cuts (Waller et al., 2002). Fifty teliospores were measured (width and length) (Anikster et al., 2004) using a compound microscope (Olympus CX 31).
Source and maintenance of plants
New P. nodiflora individuals of each variety were obtained to establish rust cultures with isolates of different locations and to conduct pathogenicity tests. Healthy stem cuts with three to four nodes (with leaves) were buried in 10 cm-diameter plastic pots containing steam sterilized soil and organic substrate mixture (1:1, w/w) leaving two nodes over the soil surface. Phyla nodiflora propagules were grown for 15 d at 25°C and 100% relative humidity (RH) (humid chamber), and thereafter at 25°C and 60% RH until the four-five leaf stage.
Rust inoculation methodology
The leaf disc inoculation method proposed by Morin et al. (1993) was used for all inoculation experiments. The host plants (3 months old) from which leaf discs were collected were P. nodiflora var. nodiflora (350), P. nodiflora var. reptans (264), and P. nodiflora var. reptans (205). Each leaf disc was covered by a mass of spores (telia) of the rust 5 mm in diameter. Ten circular discs of leaf with telia (3-4 weeks old) were placed on the surface of 2% water agar (WA) in a 9 cm diameter Petri dish base, with the telia on the side away from the water agar. The base of the dish was inverted and fixed to the top of a plastic pot with holes. Three young, healthy plants of P. nodiflora were placed in a plant pot. The pot with the plants was sealed to the pot containing the telia inverted with masking tape and incubated at 20°C for 48 h in the dark. The inside of the pots as well as the plants was sprayed with a fine mist of sterile distilled water. The pot chambers were then placed inside an inoculation chamber. This consisted of a cube shaped polyethylene boxes with the floor covered with water-soaked newspaper to provide around 100% RH.
After 48 h, the plants were transferred to the glasshouse (24±2°C, 12-h dark/12-h light (fluorescent, 1400 Lux) regime, at around 75% RH), until telia started to develop. For conservation purposes, infected leaves collected from both field surveys and pathogenicity tests were preserved inside paper bags at 3±0.5°C with silica gel to avoid the germination of teliospores.
Isolation and maintenance of rust isolates
Rust cultures were established from a single telium (Thomas et al., 2021) from rust-infected material collected at sites 205 (Salta province), 264 (Entre Rios province) and 350 (Formosa province) (Supplementary material). A single telium isolate was obtained using the leaf disc method. The single telium isolate was used to inoculate a plant of the same origin of the telium (site where the rust was collected). Healthy P. nodiflora plants were inoculated monthly with P. lantanae to ensure a continuous supply of mature telia throughout the experiments.
Biology of P. lantanae
Morphological characterization and microscopy
For light microscope observations, a drop of water was placed on a slide, the sori were scraped with a sterile needle and the teliospores were transferred into the drop of water. A coverslip was placed over it.
An Olympus SP350 camera was attached to the microscope eyepieces and photos were taken to illustrate the morphology of the telia and teliospores and characterize the pre-infection development (i.e., from teliospore to basidiospore production) of the rust fungus.
For scanning electron microscope observations, a 2.5% glu-taraldehyde fixation was performed in 0.067 M phosphate buffer (pH 7.2) and then washed with phosphate buffer (15 min, 3 times). Gradual dehydration was conducted with alcohol and acetone and then critical point drying was done (E3000, Polaron) using CO2. The samples were mounted in stubs. Finally, gold was evaporated (300 Á) using an Argon plasma metal evaporator (91000 Model 3, Pelco). Microphotographs were taken with a LEO EVO 40 scanning electron microscope at 7.0 kV potential (Mercer & Birbeck, 1972).
Germination studies
Effect of temperature on teliospore germination and basidiospore formation
Twenty days after teliospores had developed on plants, they were scraped from leaves bearing mature telia and further suspended in 5 ml of sterile distilled water (SDW). Aliquots of 0.10 ml were transferred to 9 cm diameter Petri dishes containing water agar (WA) medium were incubated at 10, 15, 20, 25, and 30°C in several growth chambers, one for each temperature. The Petri dishes were wrapped in aluminum foil to exclude light and sealed with adhesive tape. Teliospore germination was examined after 4, 7, 11, 20, 24, and 48 h of incubation. A completely randomized factorial design was used (N=90, n=3). The Petri dish was placed under the compound microscope (Olympus CX 31) at (16x and 40x of lenses eyepiece and objective) 640x, and germination percentages were recorded by randomly selecting 50 teliospores per replicate. A teliospore was considered germinated when the germ tube was observed. Basidiospore formation was determined as the percentage of teliospores producing basidiospores (i. e., that has at least one basidiospore in the metabasidium or next to it) (Morin et al., 1992b) at 10, 15, 20, 25, and 30°C after 24 h of incubation. Pictures of the Petri dishes were taken using a camera (Olympus SP 350) attached to the Olympus CX 31 microscope. Dishes were sampled destructively.
Effect of heat shock on teliospore germination and basidiospore formation
An experiment was performed in order to test the hypothesis that a heat shock during incubation (i.e., high temperature exposure) would influence the capacity of teliospore germination and/or basidiospore formation. Spores were exposed to three different incubation temperature regimes (3 h at 30°C + 21 h at 20°C; 6 h at 30°C + 18 h at 20°C; 24 h at 20°C). Teliospore germination and basidiospore formation were registered after 4, 7, 11, 20, and 24 h of incubation using the same procedure described above. A completely randomized factorial design was used (n=3) (n: number of replicates).
Effect of teliospore age on germination capacity
Final germination was assessed on WA after 48 h of incubation at 20°C after different periods of storage: 2, 5, 7, 12, and 18 months. Storage time was defined as the time between the harvest of the teliospores until the end of storage. The leaves with telia collected from inoculated plants were kept inside paper bags with silica gel in a refrigerator at 3.0±0.5°C. A completely randomized factorial design was used (n=3).
Pathogenicity tests
Pathogenicity tests determine the ability of a pathogen to provoke diseases. These tests are essential studies when a rust is proposed as a potential biocontrol of a weed (Fourie & Wood, 2018). The tests were conducted to confirm if P. lantanae could infect the three varieties of P. nodiflora. Also, the level of in vitro damage was determined.
Telia collected from fields and obtained from the laboratory plants were used for inoculations. Three experiments were designed. Telia taken from plants of P. nodiflora var. nodi-flora (site 350) and from P. nodiflora var. reptans (from sites 264 and 205) (see Supplementary material) were inoculated on plants of the three varieties (plants from sites 205, 264, 345, 350). Those plant specimens inoculated with telia of the same origin, were considered as positive controls for the pathogenicity test.
Pathogenicity tests were performed using the leaf disc method (Morin et al., 1993). Infection was checked daily and ranked using categories proposed by Ellison et al. (2008): 0 - without macroscopic symptoms; 1 - chlorosis; 2 - between 1-5 telia on leaves and stems, localized infection; 3 - more than 5 telia, until semi-systemic infection.
Statistical analysis
Germination percentages and basidiospore formation data were analyzed with ANOVA followed by a mean comparison test (protected LSD Fisher, P<0.05). Analyses of variance were performed using InfoStat software (Di Rienzo et al, 2013).
Results and discussion
Field surveys and collection of rust isolates
Puccinia lantanae was found only in locations from Argentina (Entre Rios, Formosa, Jujuy, and Salta). None of the populations of P. nodiflora in Bolivia or Chile showed symptoms of rust infection. To give a definitive conclusion on the distribution of the rust infecting Phyla in South America, it would be necessary to make more extensive collecting. The rust was observed only in five sites. Its prevalence was low (2%). Mostly it was collected on plants belonging to the var. reptans (four sites) and it was found infecting P. nodiflora var. nodiflora in only one site of the several visited. The prevalence of the rust was 2%.
Telia were observed in pustules of 5 mm of diameter. On leaves, telia were mostly hypophyllous, rounded, isolated, or arranged in concentric circles causing early defoliation of infected plants (Fig. 2A). Symptoms on leaves were characterized by chlorotic spots on the adaxial side corresponding with telia on the abaxial side. Under high humidity conditions, germination of basidiospores was evidenced as a hyaline layer covering the telia (Fig. 2B).

FIGURE 2 Telia of Puccinia lantanae. A) Infection symptoms in the field, B) Hyaline formation over telia of an infected leaf, C) One-celled and two-celled teliospores, D) Pedicel eccentrically inserted, E) Germinated basidiospore, F) Germ pore, G) Two-celled teliospores form two germ tubes, H) Ramification of germ tube, I) Metabasidia with conical sterigma. Bars: C and D: 30 µm, E: 10 µm, F: 2 µm, G: 20 µm, H: 15 µm, I: 3 µm.
Biology of Puccinia lantanae
Morphological characterization and microscopy The rust produced one- and two-celled golden-brown teliospores (Fig. 2C). The one-celled were ovoid to ellipsoidal, 17-28.5 x 13.5-23 µm. The two-celled spores were ellipsoidal to clavate, constricted at the septum, 23-36 x 12-23 µm, with thick walls, 1.5-2 laterally, and 5-7 at the apices. Two-celled spores with an oblique septum were rarely observed. In all the teliospores, the pedicel was persistent, sometimes eccentrically inserted (Fig. 2D). A germ pore was often clear on the distal end of the cells (Fig. 2F). When germinating, two-celled teliospores formed one or two germ tubes as observed in other Puccinia species (Alexopoulos et al., 1996) (Fig. 2G). Occasionally, germ tubes ramified, but these did not develop metabasidia (Fig. 2H). Basidiospores were hyaline, ellipsoidal to shortly oval (Fig. 2E).
The commonest teliospores (about 75-80% of the population) were one-celled. Thomas et al. (2021) also considered this type as the dominant one on Lantana camara, recording abundances of up to 96%. The one-celled spores showed the same mean size as the teliospores formed by the same rust species on L. camara, but the two-celled spores were narrower than those measured on that host (Thomas et al., 2021). The constriction at the septum was reported by Barreto et al. (1995) also in teliospores developing on L. camara. Therefore, the rust species of the present research was confirmed through morphological studies.
Germination studies
Effect of temperature on teliospore germination and basidiospore formation
Two processes were observed, (i) germination of teliospo-res with germ tube and (ii) four-celled metabasidia (Fig. 2I), and formation of basidiospore. Not all the teliospores germinated and not all the germ tubes developed a meta-basidium with basidiospores.
Teliospore germination was influenced predominantly by temperature. As observed in Figure 3, the highest germination values were registered at 20°C at each incubation time (P<0.05). The same result was obtained by Thomas et al. (2021), working with an Amazonian pathotype of P. lantanae on L. camara. According to Thomas et al. (2021), the maximum infection on L. camara was obtained at 20°C. In the present study, the lowest germination figures were obtained at 30°C, while no infection was found on L. camara at 30°C (Thomas et al., 2021). The germination started over a period ranging between four and 7 h of incubation (Fig. 3). Koutsidou (personal communication, August 21,2020) obtained similar germination rates working with the Amazonian isolate of P. lantanae from L.camara.
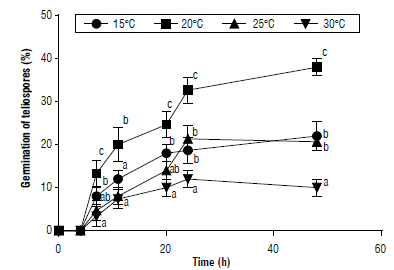
FIGURE 3 Germination of teliospores of Puccinia lantanae at different constant temperature regimes as a function of incubation time. Different letters indicate statistical differences among means (protected LSD Fisher test, P<0.05) at each incubation time. No teliospore germination was registered at 10 C. Bars show standard error.
The percentage of teliospores that produced basidiospores was determined at 10, 15, 20, 25, and 30°C (Fig. 4). No basidiospores formed at 10°C. At 20°C most of the teliospores formed metabasidia and produced basidiospores. Basidiospore development was inhibited or prevented by extreme temperatures. As observed in Figure 4, the optimum temperature for basidiospore formation was 20°C. Wide metabasidia with long sterigmata and long germ tubes did not form basidiospores. Similarly, Morin et al. (1992a) observed the branching of the germ tube, long germ tubes and wide basidia while working with Puccinia xanthii Schw. Abnormal germination can occur when telia are exposed to either sub or supraoptimal temperatures. However, the nuclear state of the mycelium which is formed from the long germ tubes needs to be addressed to establish its effective infection capacity.
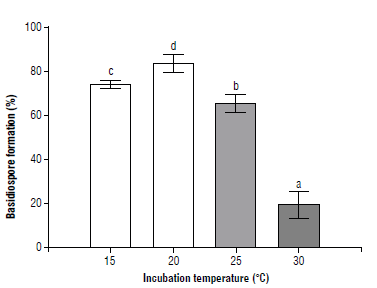
FIGURE 4 Basidiospore development of Puccinia lantanae at different constant temperature regimes after 24 h of incubation. Different letters indicate statistical differences among means (protected LSD Fisher test, P<0.05). Bars show standard error (n=3).
After 24 h of incubation at 20°C, four conical sterigma developed (Fig. 2I). Most of the basidiospores germinated while still joined to the sterigma or close to it as a common cytological phenomenon in WA.
Effect of heat shock on teliospore germination and basidiospore formation
The effect of high temperatures on the germination of teliospores was evaluated. Teliospore germination and basidiospore formation were influenced by the interaction between temperature and incubation time. Both heat shock treatments significantly reduced teliospore germination (75% in average) and basidiospore formation (64% in average) compared to the control (Figs. 5-6). Basidiospores formed in a sequential manner under high humidity conditions (Fig. 6) as well as in P. araujiae, thus, increasing the period over which the inoculum is produced and is available for new infections to take place (Anderson et al., 2016). Similar observations were reported by Morin et al. (1993) in P. xanthii.
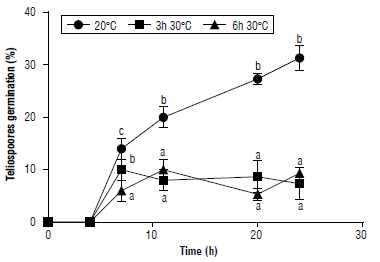
FIGURE 5 Effect of heat shock on germination of teliospores of Puccinia lantanae. Teliospores were incubated at 20°C for 24 h (control), 3 h at 30°C + 21 h at 20°C, or 6 h at 30°C + 18 h at 20°C. Different letters indicate statistical differences among means (protected LSD Fisher test, P<0.05) at each incubation time. Bars show standard error (n=3).
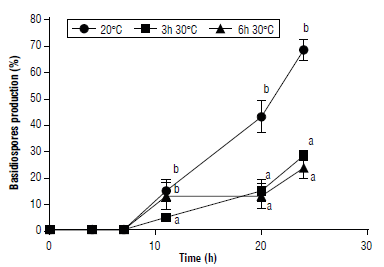
FIGURE 6 Effect of heat shock on formation of basidiospores of Puccinia lantanae. Teliospores were incubated at 20°C for 24 h (control), 3 h at 30°C + 21 h at 20°C, or 6 h at 30°C + 18 h at 20°C. Different letters indicate statistical differences among means (protected LSD Fisher test, P<0.05) at each incubation time. Bars show standard error (n=3).
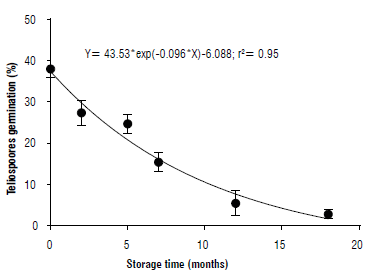
Effect of teliospores storage on germination
Storage on teliospore germination showed a negative exponential pattern (Fig. 7). A 50% reduction on germination was recorded after 7.2 months of storage at 3.0±0.5°C.
Pathogenicity tests
The first symptoms of P. lantanae on P. nodiflora plants were visible after 15-20 d. Sporulation occurred mainly on the lower surface of the leaves. The rust caused significant damage to P. nodiflora, infecting leaf, petiole, and stem tissues, resulting in cankering and stem die-back as premature leaf drop.
The different symptoms that the three varieties of Phyla nodiflora plants developed after being inoculated with teliospores from different sample sites are shown in Table 2. Inoculation was considered successful when plants exhibited low or high levels of infection (Reaction 2 and 3). Only the Puccinia lantanae isolate R350n ("Formosa isolate") produced infection on all the varieties of P. nodiflora. Phyla nodiflora var. nodiflora was the variety that exhibited most resistance to infection. The rust isolates R264r ("Entre Rios isolate") and R205r ("Salta isolate"), both from P. nodiflora var. reptans, were successfully inoculated on P. nodiflora var. minor and P. nodiflora var. reptans. These rust isolates would be potential candidates for biocontrol of the target weed.
TABLE 2 Results of the inoculations with Puccinia lantanae on Phyla nodiflora (leaf disc method).
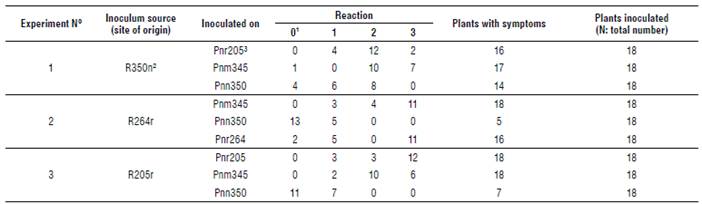
1 Reaction: (0) plants without symptoms, (1) chlorotic spots, (2) low level of sporulation (localized infection, 1-5 pustules) and (3) high level of disease (more than 5 pustules until semisistemic infection).
2 Strain of the fungus. For example: R350n means that the strain of the rust (R) was originally found in site 350 on var. nodillora plants.
3 Inoculated plants, for example: Pnr205 means that Phyla nodillora var. reptans was obtained from site 205.
N: 162
Conclusions
The rust Puccinia lantanae exhibited pathogenicity on three varieties of Phyla nodiflora, including P. nodiflora var. minor, which is a weed with serious impact in Australia. However, some features of the fungal biology could alter its effectivity. The rust showed a mesophilic behavior; higher temperatures negatively influenced both the germination of teliospores as the development of basidiospores. In addition, aging also affected teliospores, diminishing their capacity of germination after five months of storage. Of the three isolates of P. lantanae studied, two are promising candidates for biocontrol of the weed but one should be disregarded as it infects P. nodiflora var. nodiflora, which is native to Australia. This study demonstrates that there are differences between isolates of the same pathogen, which is why in-depth genetical studies of potential agents are necessary as part of a biocontrol study.