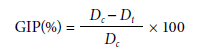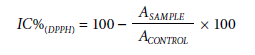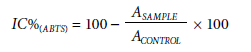Introduction
It is estimated that at least 15% of losses during seed storage occur due to product contamination by insects and fungi (Silva et al., 2021). Extracts and essential oils from plants have demonstrated ability to inhibit the action of phytopathogens or the production of mycotoxins; these promising biofungicides could mitigate the use of chemical pesticides (Mohr et al., 2017). This is due to the presence of compounds from the secondary metabolism of plants. These chemical compounds, such as terpenes, phenolics, and alkaloids are important biological agents that can play antifungal roles against species of the genus Aspergillus, Rhizopus, Penicillium, Colletotrichum as well as antioxidant roles (Elisée et al., 2020).
Natural plant-based products are a more appropriate strategy in agricultural management when compared to commercial fungicides, as they are biodegradable, prevent pathogen resistance, and are less harmful to human health (Chowdhary et al., 2018). However, the composition and activity of the bioactive components of plants vary according to genotype, geographic location, and vegetative stage, as well as method, solvent and temperature used in the extraction process (Onyebuchi & Kavaz, 2020). The extraction of secondary metabolites can be performed using a fractionation technique with solvents of different polarities to concentrate chemical groups in distinct fractions with completely different chemical characteristics in a single plant (Mann, 2012).
Ocimum gratissimum L., popularly known as clove basil, is an aromatic herb that belongs to the Lamiaceae family and is found in South America, Africa, and Asia (Mohr et al., 2017). It is used as a food condiment and in folk medicine; the infusion of leaves is prepared for the treatment of fever, flu, and kidney problems (Matos, 2007; Penido et al., 2016). It has a diversity of chemical groups with proven biological activities due to its anti-inflammatory (Dzoyem et al., 2021), insecticidal (Benelli et al., 2019), antibacterial (Hamma et al., 2020), antioxidant (Onyebuchi & Kavaz, 2020) and antifungal properties against several species of fungi, among them Aspergillus niger, Botryodiplodia theobromae, Rhizopus stolonifer, Fusarium oxysporium, Penicillium expansum, and Colletotrichum spp. (Uchegbu et al., 2019).
The chemical profile of these compounds includes the presence of flavonoids, tannins, sterols, terpenoids, saponins, and alkaloids (Hamma et al., 2020). Previous studies have shown that the ethanolic extracts and essential oils obtained from O. gratissimum leaves are rich in phenolic compounds with antioxidant and antimicrobial action against various pathogens (Dambolena et al., 2010; Elisée et al., 2020; Onyebuchi & Kavaz, 2020). The leaves are rich in essential oils, whose main components contain eugenol, thymol, and linalool, with antimicrobial activities already documented in the literature (Faria et al., 2006; Mohr et al., 2017).
Most studies on the control of phytopathogens have focused on the biological properties of essential oils; there are few data based on extracts and their fractionation from O. gratissimum leaves against phytopathogens (Dambolena et al., 2010; Mohr et al., 2017; Elisée et al., 2020). Therefore, the aim of the present study was to evaluate the in vitro antifungal and antioxidant capacity and the chemical profile of the most active samples from O. gratissimum leaves.
Materials and methods
Collection and botanical identification of the plant species
Collection and botanical identification of O. gratissimum L. leaves were done at the Engenheiro Agronomo Reginaldo Conde (FERC) Experimental Farm of the Institute for Research, Technical Assistance and Rural Extension of Espírito Santo (INCAPER), Viana, Espírito Santo, Brazil, in October 2019.
Preparation of the ethanolic extract and its fractions
The plant extract was prepared at the Laboratory of the Federal Institute of Espírito Santo (Ifes), Vila Velha campus (Brazil). Leaves were dried in an oven with air circulation at 40°C for 24 h and then crushed. To obtain the ethanolic extract (EEtOH), leaves were macerated in 96% ethanol at 1:10 w/v ratio (dryer plant: ethanol), at room temperature and protected from light. Subsequently, the extract was filtered, and the solvent removed on a rotary evaporator (Buchi Rotavapor R-3 CH 9230 Flawil 1, Switzerland). The recovered solvent was added to the leaf residue and crushed again until the plant drug was depleted. The concentrated residue (EEtOH) obtained was stored in amber glass under refrigeration at 4°C. To obtain fractions of different polarities, part of the EEtOH was resuspended in the ethanol-water mixture v/v (8:2) and submitted to successive liquid-liquid partitions with organic solvents of increasing polarities; after total removal of solvents, the following fractions were obtained: hexane (FHex), dichloromethane (FDCM), ethyl acetate (FAce), n-butanol (FBuOH), and the aqueous residual (FAq).
Antifungal activity
Mycelial growth inhibition percentage
The experimental design was completely randomized, in a 6x3 +2 factorial scheme, with three replicates. Factor A was composed of six different extracts (EEtOH; FHex; FDCM; FAce; FBuOH and FAq) and factor B was composed of three concentrations (0.1; 5.0, and 10.0 mg ml-1), plus two additional treatments, negative control, and positive control. The negative control did not contain EEtOH or fraction and the positive control was Cercobin® commercial fungicide based on thiophanate methyl (Dimethyl 4,4'-(o-phenylene) (3-thioallophanate) at 0.8 mg ml-1.
For the in vitro antifungal activity evaluation, the agar diffusion method according to Barros et al. (1995) was used. The ethanolic extract and fractions were tested on Aspergillus sp. and Rhizopus sp., isolated from traditional bean and corn seeds and identified at the Laboratory of Diagnosis of Plant Diseases of Federal Institute of Espírito Santo (Ifes), Santa Teresa campus.
Sterilized stock solutions (15 mg ml-1) were diluted in sterilized Potato-Dextrose-Agar (PDA) medium, in a melting state, to obtain final concentrations of 0.1, 5.0, or 10.0 mg ml-1 for each sample. Then, the final solutions were poured into 5 cm diameter Petri dishes. The fungi were spiked into the center of each Petri dish. Plates were incubated in a growth chamber (BOD) at 25°C, 12 h photoperiod, until the mycelial growth of the respective fungi in the negative control treatment reached the edge of the plate. Mycelial growth was evaluated by daily measurement of the diameter (in cm) of colonies. The mycelial growth inhibition percentage (GIP) was estimated using the equation (Kordali et al., 2003):
where Dc is average mycelial diameter of the negative control (cm) and Dt is average mycelial diameter of treatments (cm).
Determination of the Minimum Inhibitory Concentration (MIC)
The most promising (due to preview antifungal action in susceptibility tests) samples from O. gratissimum leaves were selected for the determination of the Minimum Inhibitory Concentration (MIC). The MIC of the main component of both samples, eugenol, was also evaluated.
The EEtOH and the FDCM were added to PDA to make a 5 mg ml-1 stock solution. Serial dilution was performed to obtain concentrations of 5.0, 2.5, 1.25, 0.625, 0.3125, and 0.1 mg ml-1; then the agar diffusion method was performed, as previously mentioned. For evaluation of pure eugenol, a stock solution of 1.0 mg ml-1 of pure eugenol in 0.5% DMSO (dimethyl sulfoxide) was prepared; subsequently serial dilutions were performed with final concentrations of 1.0, 0.75, 0.5, 0.25, and 0.125 mg ml-1. They were then incubated for 48 h at 25°C and the fungal growth was observed. The lowest concentration, which inhibits the visible growth of the tested organism after macroscopic evaluation, was determined as Minimum Inhibitory Concentration (MIC) (Talibi et al., 2012). All determinations were tested in triplicate.
In vitro antioxidant capacity
DPPH test
The antioxidant capacity of the EEtOH and fractions using the 2,2-diphenyl-1-picryl hydrazyl (DPPH) free radical scavenging was determined according to Casagrande et al. (2007), with modifications. Five hundred µl of 250 µM DPPH ethanolic solution was mixed to 30 µl of solutions containing decreasing concentrations of the extracts in ethanol (1000, 500, 250, 125, and 62.5 µg ml-1) plus 1,000 µl of 0.1 M acetate buffer and 1,000 µl of absolute ethanol. The so-called "blank" solution was prepared with ethanol-DPPH mixture. After 30 min of incubation at room temperature, the absorbance was read against a blank at 517 nm in a spectrophotometer. The DPPH radical scavenging activity was expressed as inhibition percentage:
where ASAMPLE is the absorbance of treatments, and ACONTROL is the absorbance of control (containing all reagents, except treatment sample).
The antioxidant activity was evaluated based on the IC50 value (µg ml-1) extract concentration necessary to scavenge 50% of the DPPH free radical by linear regression using concentration values (µg ml-1) versus inhibition percentage (IC%).
ABTS test
The antioxidant capacity of the EEtOH and fractions was determined using the 2,2-azinobis (3-ethylbenzothia-zoline-6-sulfonic acid) (ABTS) free radical according to Sánchez-González et al. (2005). ABTS+● cation was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate. The mixture was stored in a dark bottle at room temperature for 16 h. The ABTS solution was diluted in phosphate buffer (pH 7.4) to an absorbance of 0.7 at 730 nm. The samples were resuspended in ethanol, generating solutions with concentrations ranging from 62.5 to 1,000.0 µg ml-1. After adding 10 µL of each sample standard to 4 ml of diluted ABTS+● solution, absorbance readings at 730 nm were performed after 6 min of reaction in the spectrophotometer. The antioxidant capacity was calculated by the inhibition percentage of the ABTS radical activity (IC% (ABTS)), according to the following equation:
where ASAMPLE is the absorbance of treatments and ACONTROL is the absorbance of control (containing all reagents except the treatment sample).
The antioxidant activity was evaluated based on the IC50 value (µg ml-1) of the extract concentration necessary to scavenge 50% of the ABTS free radical by linear regression using concentration values (µg ml-1) versus percentage inhibition (IC%).
Iron reduction assay (FRAP)
The antioxidant capacity of the EEtOH and fractions was evaluated by reducing iron (FRAP) based on the method of Sánchez-González et al. (2005), with some modifications. This method is based on the reduction of the ferric ion (Fe3+) to the ferrous ion (Fe2+) by antioxidant molecules present in the extracts and subsequent formation of a colored complex of the Fe2+ and 4,6-tripyridyl-s-triazine (TPTZ). The FRAP reagent was prepared as follows: 2.5 ml of a 10 mM solution of TPTZ in 40 mM HCl were added to 2.5 mL of FeCl3.6H2O and 25 mL of 0.3 mM acetate buffer pH 3.6. The solution was incubated at 37°C for 30 min in a water bath. For the evaluation of the antioxidant capacity, 900 µl of the previously prepared FRAP reagent was mixed with 90 µl of distilled water and 10 µl of the sample or standard. The samples were incubated at 37°C for 30 min and the reading was performed at 595 nm in a UV-Visible spectrophotometer (Biospectro SP-220). Standard solutions with different concentrations of Trolox (0.5, 1.0, 2.5, 5.0, 10.0, 15.0, and 20.0 µmol) were used for calibration. Results were expressed as µmol Trolox equivalent/g sample (TEAC - Trolox equivalent antioxidant capacity).
Analysis by gas chromatography coupled to a mass spectrometer
The analyses by gas chromatography coupled with mass spectrometry (GC/MS) were performed at the Laboratory of Chromatography of LabPetro, Federal University of Espírito Santo. The nonpolar fractions that showed the greatest biological effects were analyzed by a gas chromatograph coupled to an Agilent 7890B mass spectrometer (Agilent, CA, USA) and a model 5977A MSD mass detector with electronic ionization of 70 eV. The column used was a 30 m x 250 µm x 0.25 µm HP-5 column. The injector was set to a temperature of 290°C and the detector to 310°C.
Elution was done on a heating ramp, starting of 40°C with a heating rate of 5oC/min to 280oC, followed by a heating rate of 15oC/min to 310oC, remaining at that temperature for 10 min.
For characterization, a C10 to C40 alkane standard was used and submitted to the same chromatographic conditions. Compounds were identified through comparison with the National Institute of Standards and Technology (NIST) database library followed by comparison of literature retention rates (NIST, 2018).
Statistical analysis
Mycelial growth inhibition percentage data were submitted to analysis of variance (ANOVA). The interaction between factors was analyzed; later the Tukey test (P<0.05) was applied for the factorial group, and the Dunnett test (P<0.05) was applied for the comparison of means of the factorial group with additional treatments (positive and negative control), using Assistat 7.6 software.
The antioxidant activity was evaluated using ANOVA followed by the Tukey test (P<0.05) using Assistat 7.6 software. All experiments were completed in triplicate to ensure reproducibility.
Results and discussion
The mycelial growth inhibition percentage
Antifungal activity of the samples of O. gratissimum at different concentrations against Aspergillus sp. and Rhizopus sp. fungi is shown in Table 1. For the variable growth inhibition percentage (GIP), there was a significant interaction between the concentrations of the plant extracts tested.
TABLE 1 Mycelial growth inhibition percentage (GIP) of Aspergillus sp. and Rhizopus sp. submitted to different ethanolic extract concentrations (mg ml-1) and fractions from Ocimum gratissimum leaves.
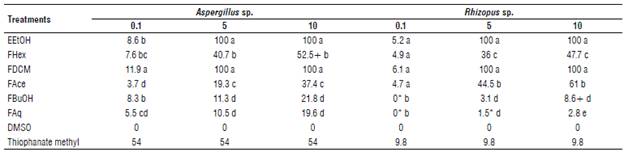
EEtOH: ethanolic extract; FHex: hexane fraction; FDCM: dichloromethane fraction; FAce: ethyl acetate fraction; FBuOH: butanol fraction; FAq: aqueous fraction; DMSO: negative control. Means followed by different lowercase letters in the column differ statistically from each other by the Tukey's test (P<0.05). Means followed by * and + do not differ statistically from DMSO and fungicide treatments, respectively (Dunnett; P>0.05).
The results indicate that EEtOH and FDCM at 5 and 10 mg ml-1 had high antifungal activity, with complete suppression (100%) of the mycelial growth of Aspergillus sp. and Rhizopus sp. These were the only treatments against Aspergillus sp. and Rhizopus sp. with antifungal activity higher than treatment synthetic fungicide, in 0.8 mg ml-1. Among the polar fractions, FAce at 10 mg ml-1 was the most active with GIP of 37.4 and 61% for Aspergillus sp. and Rhizopus sp., respectively.
Minimum Inhibitory Concentration (MIC)
The MIC results of the most activity samples of O. gratis-simum and eugenol are found in Table 2. The MIC ranged from 0.625 to 1.25 mg ml-1. The samples FDCM exhibited the lowest MIC with a value of 0.625 mg ml-1 for Aspergillus sp. and Rhizopus sp., while for EEtOH, the MIC for Aspergillus sp. was 1.25 mg ml-1 and for Rhizopus sp. it was 0.625 mg ml-1. Preliminary chemical analysis showed eugenol as major compounds in the sample of the EEtOH and FDCM, MIC was done with eugenol pure, and the result was 0.125 mg ml-1.
Antioxidant capacity
The antioxidant potential of O. gratissimum extracts was studied by means of synthetic radical tests, DPPH and ABTS, and by the FRAP assay, as with the results shown in Table 3. The antioxidant potential of extracts by DPPH and ABTS consisted of the ability to eliminate free radicals by donating a hydrogen atom or an electron. The antioxidant capacity is related to the degree of discoloration of the reaction solution with the synthetic free radical (Re et al., 1999; Sousa et al., 2007).
The EEtOH partition exhibited the highest antioxidant capacity for both DPPH and ABTS assays, followed by nonpolar fractions, FHex and FDCM, respectively. Low potential was identified in polar fractions, with FAce significantly lower among all extracts evaluated in the DPPH and ABTS assays. FAq was the only extract that did not show significant potential for scavenging the ABTS radical. The FRAP assay defines antioxidant as any substance in the reaction medium with reducing power by donating a hydrogen atom (Duh et al., 1999). Thus, EEtOH presented the highest reducing power and as the polarity is increased, the reduction capacity of samples is smaller, with FAq showing the lowest value.
TABLE 3 Antioxidant activity by DPPH, ABTS, and FRAP of the ethanolic extract and fractions from Ocimum gratissimum leaves.
| Treatments | DPPH IC50 (pg ml1) | ABTS IC50 (pg ml1) | FRAP (TEAC) |
|---|---|---|---|
| EEtOH | 371.1±2.98 e | 182.43±1.1 e | 262.39±3.61 a |
| FHex | 405.60±3.21 e | 325.86±3.49 d | 229.88±1.65 b |
| FDCM | 707.11±2.75 d | 370.00±1.76 c | 111.51±5.03 c |
| FAce | 2088.33±13.52 a | 641.06±8.05 a | 39.62±1.63 e |
| FBuOH | 905.96±5.64 c | 495.66±5.63 b | 64.13±2.29 d |
| FAq | 1748.52 ±8.36 b | 4816.50±20.35 ns | 27.32±1.9 f |
EEtOH: ethanolic extract; FHex: hexane fraction; FDCM: dichloromethane fraction; FAce: ethyl acetate fraction; FBuOH: butanol fraction; FAq: aqueous fraction; ns: not significant. Means followed by different lowercase letters in the column differ statistically from each other by the Tukey's test (P<0.05).
Identification by GC/MS
In the GC/MS chromatographic profile EEtOH samples and their nonpolar fractions from O. gratissimum leaves, 22 substances were identified, shown in Table 4. From chromatograms, the presence of eugenol as the major substance in all samples was observed, with the highest relative percentage (61.26%) in the FDCM fraction, followed by the EEtOH extract (59.61°%) and FHex fraction (37.65%).
The Aspergillus sp. and Rhizopus sp. fungi can accelerate the deterioration process of stored seeds (Silva et al., 2021). The present study demonstrated in vitro antifungal activity of the EEtOH and FDCM samples from O. gratissimum leaves at 5 mg ml-1 against Aspergillus sp. and Rhizopus sp. fungi associated with seeds in the storage phase, which indicates biological potential of extracts obtained from this plant. Onaebi et al. (2020), using the EEtOH at 100 mg ml-1 from O. gratissimum leaves, found reductions in the growth of Aspergillusflavus (51.93%) and Aspergillus niger (23.7%), but the same did not occur for Rhizopus delemar (0%); the difference from the present study may be due the extract preparation.
TABLE 4 Compounds identified by gas chromatography coupled to mass spectrometer from the ethanolic extract and the hexane and dichlorometha-ne fractions from Ocimum gratissimum leaves.
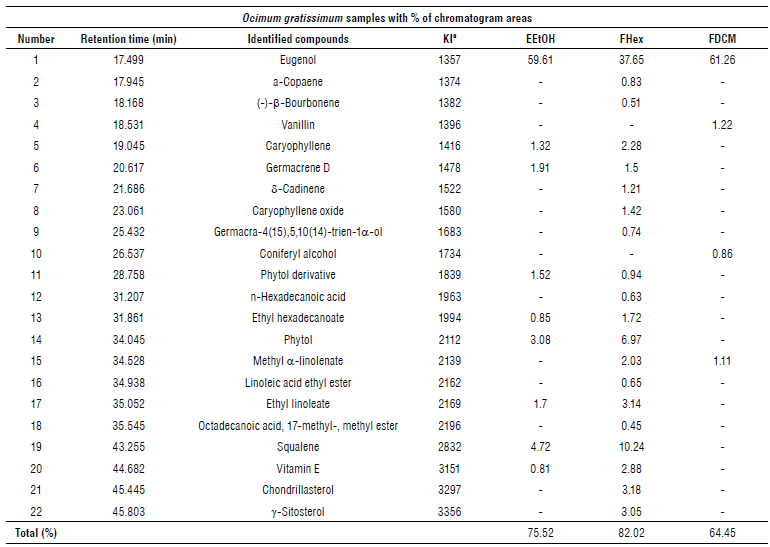
a Retention index obtained as a standard reference of v-alkanes using HP-5MS column. EEtOH = Ethanolic extract, FHex = Hexane fraction, and FDCM = Dichloromethane fraction.
The fraction FDCM and the EEtOH extract showed the best antifungal activities, and the GC/MS analysis indicates eugenol as the major compound. Eugenol is a phenylpropanoid commonly found in essential oils extracted from O. gratissimum leaves and, due to its nonpolar characteristic, it was easily identified in the less polar fractions and in the ethanolic extract in this study. Eugenol is related to several biological activities, mainly against phytopathogens (Faria et al., 2006; Dambolena et al., 2010).
There are numerous studies with essential oils of O. gratissimum; however, research for ethanolic extracts and fractions of different polarities is scarce (Zareiyan & Khajehsharif, 2022). In our experiment, the MIC results showed that eugenol has higher activity than EEtOH and FDCM samples. Ethanol is a non-selective solvent in the process of extracting compounds from plants, thus allowing for a greater chemical diversity of constituents in the extracts obtained, but at low concentrations. On the other hand, fractions obtained from the fractionation process with selective solvents of different polarities have higher concentrations and lower diversity of phytochemicals and are more selective in terms of polarity, thus the DCM sample had higher concentrations of the compounds with antifungal activity. Nwofor et al. (2021) presented the MIC of 100 mg ml-1 for methanolic extracts of O. gratissimum against Penicillium citrinum, Aspergillus aculeatus, As-pergillus fumigatus, Curvularia kusanol, and Absidia spp.
The EEtOH showed the highest antioxidant capacity among samples used in the DPPH, ABTS and FRAP methods. Ouyang et al. (2013) showed antioxidant activities of methanolic extracts and ethyl acetate fraction from O. gratissimum leaves using the same methods as in this study.
In our study, the non-polar fractions demonstrated more antioxidant capacity than polar fractions. This could be explained by the presence of eugenol substance in all samples and other antioxidants like squalene and vitamin E.
The results of this study confirmed that O. gratissimum extracts have antifungal and antioxidant activity, but the type of solvent can interfere with the chemical composition and biological properties. The mechanism of action is likely related to the presence of eugenol. In addition, extracts can scavenge free radicals, which could reduce seed deterioration during storage. However, it is still necessary to verify the in vivo efficacy and improve the activity with the release of the active ingredient at the specific site of action.