Introduction
Soil's ability to provide mineral nutrients to plants depends on its physical, chemical, and biological characteristics and their interactions (Ma et al., 2022). Attention to the quality of soil has grown and its rational management has increased its productive capacity and guaranteed the quality of the product resulting from the uniformity of planting (Brown et al, 2022; Bulut, 2022). The amount of secondary metabolites found in plant species directly relates to soil properties, and their characterization is of fundamental importance to maintain production quality and avoid significant chemical variations of natural products derived from plants (Diomande et al, 2015).
Solanum paniculatum L. (Solanaceae) is a widely studied plant; it grows in the tropical Americas and is used for culinary purposes and in folk medicine (Vieira et al, 2010; Saqueti et al., 2022). S. paniculatum is common in almost all of Brazil, where this plant is known as "jurubeba", with fructification throughout the year, abounding in bare lands, roadsides, and degraded pastures (Macedo-Costa et al., 2017). Given its medicinal, antidiarrheal, anti-inflammatory, antimicrobial, anti-ulcer, antioxidant, and antitumor properties (Tenório et al., 2016; Souza et al., 2019), it has been recognized as a phytotherapy medicine by the Brazilian Pharmacopoeia, and the chemistry of S. paniculatum fruit and leaf has been well studied (Endringer et al., 2010; Gazolla et al, 2019). Previous chemical studies of various parts of S. paniculatum including roots, fruits and leaves have revealed the presence of steroidal alkaloids, steroidal glycoalkaloids, steroidal saponins, terpenes, and phenylpropanoids (Vieira Júnior et al, 2015; Valerino-Díaz et al, 2018).
Although the plant has been widely exploited for therapeutic and food purposes, the studies of mineral nutrients in S. paniculatum have been neglected. There are many studies on nutrients and soil fertility with other Solanum species such as the well-known vegetables tomato (S. lyco-persicum), potato (S. tuberosum), eggplant (S. melongena) and bitter tomato (S. aethiopicum) (Han et al., 2021; Can et al., 2022). Thus, this study was directed towards the determination of the main mineral nutrients in the roots of S. paniculatum and searched to establish a correlation between the contents of mineral nutrients in the roots and in the surrounding soil.
Materials and methods
Plant collection and preparation
Roots from adult S. paniculatum L. plants (n = 5) separated by a minimum distance of 3 m were randomly collected in an Atlantic Forest fragment located in the city of Camaragibe, in the state of Pernambuco in Northeastern Brazil (7°58'36" S and 34°58'51" W, tropical monsoon climate, according to the Köppen criteria). An annual average maximum temperature of 29.1°C, average annual relative humidity of 83.9%, and average precipitation of 92.1 mm were recorded for the collection site of roots and soils where there is low precipitation in summer and high precipitation in winter. On the day of collection, a temperature of 30.2°C, relative humidity of 74.5%, and precipitation of 7.2 mm were recorded for the collection site. The species was identified by the Instituto Agronômico de Pernambuco - IPA and one accession was deposited in the Herbário Dárdano de Andrade Lima with number 88503 and registered at National System of Genetic Heritage Management and Associated Traditional Knowledge platform under reference number A1096D6. The roots were washed with deionized water and dried on paper towels and then in a circulating air oven for a period of 72 h at a temperature of 40°C. The oven-dried roots were crushed and sieved with a 10-mesh sieve.
Soil sampling
Soil samples (n = 5) were collected simultaneously with the roots at a depth of 0-20 cm in the area around the roots of S. paniculatum and classified as latosols according to Brazilian Soil Classification System (Santos et al, 2018) and typically classified as oxisols (USDA soil taxonomy). Soil samples were air dried, ground and put through a 2 mm mesh before being analyzed. A temperature of 30°C, mean precipitation of 67.2 mm, mean relative air humidity of 74.5% and mean atmospheric pressure of 1012.1 mbar were registered on the day of soil collection (Pernambuco Water and Climate Agency (Agência Pernambucana de Águas e Clima, n.d.) and Brazilian National Institute of Meteorology (Instituto Nacional de Meteorologia do Brasil, n.d.)).
Soil analysis
Soil pH was determined potentiometrically (Bel Engineering PHS3BW, Brazil) in a 1:2.5 soil:deionized water suspension after 1 h of contact, with suspension agitation before reading (Beretta et al., 2014). Exchangeable calcium and magnesium were determined by complexometric titration with EDTA (0.0125 mol L-1 ) using as extractor KCl (1 mol L-1) in a proportion 1:10 solution:extractor, while exchangeable aluminum (AlKCl) was determined by acidity titration with NaOH (0.025 mol L-1) using the same extractor KCl (Shelke & Sheikh, 2020). Exchangeable potassium and sodium were carried out in a double acid solution of HCl (0.05 mol L-1) + H2SO4 (0.0125 mol L-1) and determined by flame photometry for potassium (Benfer, BFC 150, Brazil) and by colorimetry for phosphorus (Agilent, 8453 United States), with soil mixed with a solution in a proportion: 1:10 (Brondizio & Moran, 2009). Extractive acidity was measured using the calcium acetate method. A solution of calcium acetate (0.5 M) was used for extraction, buffered to a pH 7.0 in a proportion 1:20 and titrated with a dilute solution of NaOH 0.025 mol L-1 (Brondizio & Moran, 2009). The sum of bases (SB) was calculated in cmol kg-1 according to the following expression: SB = Ca2+ + Mg2+ + K+ + Na+ (Martins et al., 2011) while cation exchange capacity (CEC) was calculated in cmol kg-1 (pH 7.0) using the expression: CEC = SB + H+ + Al3+ (Briedis et al., 2012). Base saturation (V, %) was determined according to the following expression: V, % = (SB/CEC) x 100, while aluminum (m, %) saturation and sodium (SNa) saturation were calculated according to the following expressions: m = 100 x Al3+/SB + Al3+ and SNa =100 x Na CEC, respectively.
Root nutrient analysis
The concentrations of total cadmium, copper, iron, potassium, manganese, sodium, zinc, magnesium, and calcium in the root samples were determined using an atomic absorption spectrophotometer (Varian AA240FS, Mul-grave, Australia) operated under the following parameters: wavelengths for Cd (228.8 nm), Cu (324.7 nm), Fe (248.3 nm), K (769.9 nm), Mn (279.5 nm), Na (589.6 nm), Zn (213.9 nm), Mg (285.2 nm), and Ca (422.7 nm); fuel/oxidizer ratio (2/15.3); lamp current 5 mA; air/acetylene and acetylene/ nitrous flame; slit width 0.2 nm; acetylene flow rate 2.0 L min-1; air flow rate 13.5 L min-1; nebulizer flow rate 4.0 mL min-1; and flow time 5 s.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD) and one-way analysis of variance (ANOVA). The Shapiro-Wilk normality test and Pearson's correlation were used to establish relationships among studied parameters. Statistical significance was considered at P<0.05 and <0.01. The software BioEstat 5.3 was used to statistical analysis.
Results and discussion
The soil had an acidic pH of 4.6, with high Ca2+ content, medium Mg2+ content, low K+, Na+ and P contents (Tab. 1) (Santos et al., 2021).
TABLE 1 Results for soil fertility analysis.
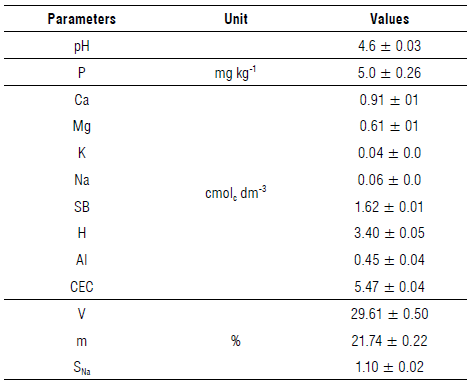
SB - sum of bases, V - base saturation, m - aluminum saturation, Sna - sodium saturation. Values expressed with mean ± standard deviation.
The values of V, SB, and SNa were low, while the m and CEC values were medium, classifying the soil as poor (Luz et al., 2002). In acidic soils, the incorporation of nitrogen can be inhibited due to a reduction in the rate of conversion of ammonium to nitrate. This explains studies reporting high levels of phenolic compounds (Tenório et al., 2016) accumulated in plants growing in this type of soil. In general, the effects of mineral nutrients on the levels of shikimic acid derivatives, especially derivatives of cinnamic acid, hydrolysable and condensed tannins, are well documented; deficiencies in nitrogen, phosphorus, sulfur, and potassium generally result in higher concentrations of these metabolites in plants (Gobbo-Neto & Lopes, 2007; Kumar & Goel, 2019). Aran et al. (2014) demonstrated that the addition of lime to soil influenced the height and stem diameter of S. paniculatum, with an increase of 32.63% and 21.16%, respectively, in relation to the treatment without lime. These results were attributed to an increase in soil pH when OH- and CO3 2- ions interact with the H+ and Al3+ ions retained in the soil colloids and neutralize them. In turn, this provides increased solubility of Ca2+ and Mg2+ ions in soil, greater mineralization of soil organic matter and, consequently, increased availability of nutrients for plants, thus, favoring their growth and development.
The analysis of the mineral content in the roots of S. paniculatum found a greater concentration of calcium, representing 92.84% of the analyzed mineral nutrients (Tab. 2), which can be attributed to the calcium carbonate precipitation process on root surfaces (Canakci et al., 2015).
TABLE 2 Content of mineral nutrients in S. paniculatum roots.
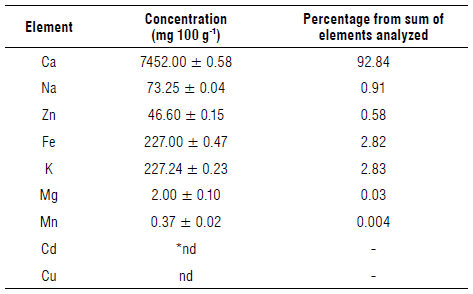
*Not detected. Values expressed with mean ± standard deviation.
Calcium is important for plants as a constituent of cell walls and membranes, contributing to the structure of cells and the upholding of physical barriers against pathogens (Waraich et al., 2012; Thor, 2019). When there is a calcium deficiency, cell membranes begin to disintegrate, cell compartmentation is disrupted, and the binding of Ca with pectin in the cell wall is affected, resulting in cell decomposition (White & Broadley, 2003).
A correlation analysis of the mineral nutrient contents in the roots of S. paniculatum was performed (Tab. 3).
TABLE 3 Pearson's correlation coefficients found between the contents of mineral elements in the roots of S. paniculatum.
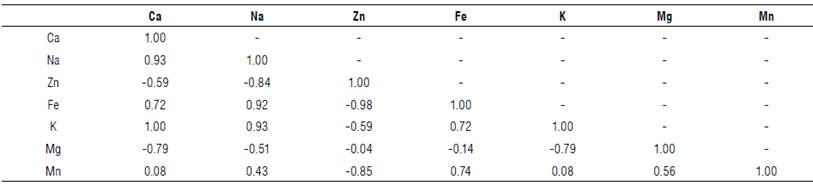
All correlation is coefficients at the P<0.05 and <0.01.
The Pearson's correlation between the contents of mineral nutrients revealed a positive correlation between Ca, Na, Fe and K, and between Fe and Mn, which might indicate that as the plant absorbs a mineral nutrient it carries another one (Lira et al., 2021).
Negative correlations were observed between Zn and Ca, Na, Fe, K, Mn as well as between Mg and Na, K and Ca. Thus, the cultivation of S. paniculatum should avoid soils rich in zinc, which in high concentrations may interfere with the concentration of Ca, Na, Fe, K and Mn by the species (Treter et al., 2021), which can affect the development of the plants. The same effect is explained for Mg, which, apparently, hinders the absorption of Na, K and Ca for the species under study.
A study carried out by Emerenciano et al. (2013), using Pearson's correlation analysis, observed that the extent to which Azadirachta indica absorbs Fe, Cu, Zn and K determines the synergistic effect of absorption of other mineral nutrients. For the metals K, Ca and Mg, negative correlations were observed, indicating that when a fertilizer rich in potassium is added, there is a reduction in the concentration of Ca and Mg. Iron accounted for 2.82% of the mineral nutrients; in plants, iron is an essential nutrient required for various cellular processes such as respiration, chlorophyll biosynthesis, and electron transport in photosynthesis. Considering that calcium was the major nutrient found in the S. paniculatum roots as well as in the surrounding soil, a Pearson correlation analysis between the calcium present in the soil and the mineral nutrients absorbed by the roots was performed (Tab. 4).
TABLE 4 Pearson's correlation coefficient for soil calcium and root nutrient minerals.
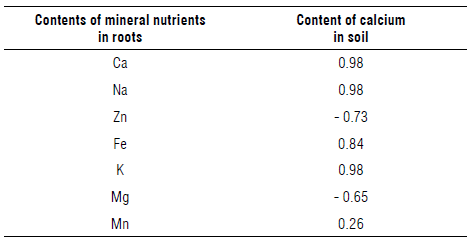
All correlation is coefficients at the P<0.01.
The results corroborated the hypothesis of a correlation between the contents of mineral nutrients in the roots, where there is a negative correlation between the Ca and Mg (r = - 0.65). This confirms competition between calcium and magnesium, with a preference for calcium by the plants. During the absorption by roots, nutrients as Ca, Mg, and K can be strongly antagonistic, and Mg is the least taken up nutrient (Farhat et al., 2016). In sunflower plants grown under insufficient Mg supply, an increase in Ca and K absorption was observed, indicating competition between these nutrients (Lasa et al., 2000). Magnesium uptake increased also during the Ca stress periods reflecting the antagonism between Ca and Mg (Taylor & Locascio, 2004). A negative correlation between the Ca2+ concentration in soil and the Zn concentration in roots (r = -0.73) can be associated with high calcium content in the soil, as previously reported for rice seedlings, where zinc absorption was reduced by about 90% with the addition of calcium and magnesium (Sadana & Takka, 1983). Furthermore, the positive correlation between the soil Ca2+ contents and the contents of Na, Fe, K, Ca in the S. paniculatum roots was confirmed, where in the case of calcium, there could be a simultaneous absorption with these other minerals.
Conclusion
S. paniculatum L. were found abundant in calcium-rich soils, with their roots showing a positive correlation for calcium contents with iron, sodium, potassium and a negative correlation with calcium, magnesium, and zinc. The results contribute to studies of S. paniculatum cultures aiming at the production of herbal medicines and food products, as the growth and development of plants depend on the mineral nutrients absorbed by roots from the soil.














