Introduction
Passiflora edulis f. flavicarpa Deneger is commonly known as yellow passion fruit (López et al., 2006) in reference to the passion of Christ. The fruit has its origins in an ancestral form known in Colombia as gulupa (P. edulis f. edulis Sims), commonly known in English as round passion fruit or purple passion fruit and distributed in the Brazilian Amazon region (Lima & Da Cuhna, 2004). The center of passion fruit diversity is the Andean region (Ocampo et al. 2013). The fruit's aroma, acidity, and content of passiflorine have made it one of the most promising fruit crops in Colombia (MADR, 2006; MinTIC, 2019; Procolombia, 2021). Among all national passionflower production, passion fruit cultivation corresponds to 35% of the most cultivated passionflower plants in Colombia (MADR, 2021).
In Colombia in 2019 the passion fruit crop was mainly cultivated in the departments of Meta (35,000 t), Antioquia (32,000 t), and Huila (17,000 t) with a total annual production of 137,000 t (Treid, 2022). In Boyacá, the municipalities of Miraflores, Tenza and Covarachía were also engaged in production of this fruit that in 2021 reached 1,345 ha, with a production of 9,439 t and a yield of 14 t ha-1 for various passion fruit crops (MinTIC, 2020; MADR, 2021). During 2019, only 252 t of the fruits were exported from the 137,000 t produced nationally (MADR, 2020). Exports were mainly destined for Spain, France, and the Netherlands (MADR, 2020). Although production is high, exports are not very significant due to different adverse impacts observed on the crops, including Fusarium sp. (MADR, 2021).
Fusarium is a saprophytic filamentous phytopathogenic fungus that produces toxins such as fumonisins, trichothecenes, and zearalenone (Agrios, 1988). Species like F. oxysporum have been used to control illicit crops. That is why their dispersion is expanding throughout many parts of the world and particularly in Colombia (Jelsma, 2000; Garcés et al., 2001; Orozco & Garcés, 2007). The presence of this fungus in soils affects crops and causes a disease called Fusarium wilt (Ortiz & Hoyos, 2012). This results in losses that reach up to 100% of passionflower crops in some municipalities of the departments Cundinamarca and Antioquia (Torres et al., 1999). Worldwide losses from this pathogen in other crops, such as beans (Phaseolus vulgaris) and bananas (Musa acuminata) are seen in Indonesia (121 million USD), Taiwan (253 million USD), and Malaysia (14 million USD) (Fontagro, 2020).
So far, an evaluation of pathogen resistance has only been reported for Passiflora maliformis; and, therefore, it has been used as rootstock for passion fruit and gulupa crops (Forero et al., 2016); but it is important to search for materials resistant to F. oxysporum within the intra-specific variety of passion fruit for genetic improvements (Silva et al., 2013).
The presence of F. oxysporum in passion fruit crops not only reduces the quality of the fruits but can also lead to the total loss of the crops (Torres et al., 1999). However, some landraces may show a tolerant response to the pathogen compared to cultivars that are genetically homogeneous. For this reason, the current study aims to contribute to the characterization of passion fruit populations by evaluating the responses of some landraces and cultivated varieties against this pathogen as well as its relationship to morphological and genetic diversity.
Materials and methods
Collection of Passiflora edulis f. flavicarpa and its morphological evaluation
We collected 63 populations of P. edulis f. flavicarpa in five departments of Colombia, and we classified them as cultivars or landraces (Tab. S1). The sampling of the cultivars consisted of collecting one to five individuals per site that were registered as a single accession. The landraces were every plant that was found on the roadsides and in reserve areas and orchards and that were not part of crops. For landraces and cultivars, mature fruits were evaluated for volume (cm3), fresh weight (g), fruit shape, pulp, and seed color and by degrees Brix (°), using a 0-90% Brix Atc (± 0.2%) refractometer (Biomed Instruments). For the seeds, the following characters were evaluated: percentage germination (number of germinated seeds / number of seeds planted) * 100% (ISTA, 1976) at room temperature and germination rate at 3 months, number of seeds per fruit, and number of seed pits by cm2 (Rodríguez Melgarejo et al., 2020). For leaves, the following characteristics were determined: leaf area (leaa), leaf invagination angle (leaang), leaf petiole length (pelon). For flowers, the following characteristics were determined: sepal area (separ), floral peduncle diameter (pedd), sepal length (proleng), petal area (petar), operculum diameter (opd), androgynophore length (andleng), filament length (filleng), anther length (antleng), style length (styleng), ovary longitudinal diameter (ovalond), ovary transverse diameter (ovatrad), transverse stigma length (trastileng), and longitudinal stigma length (lonstileng) (Crochemore et al., 2003; Ángel-Coca et al., 2011; Castro et al., 2012). Based on the diversity found, 18 accessions were selected for evaluation against F. oxysporum.
Activation of F. oxysporum to produce the inoculum
The F. oxysporum was isolated from P. edulis plants and confirmed by the OCATI plant pathologist (Sr. Ricardo Mora, pers. comm.). The F. oxysporum was cultivated in a potato dextrose agar (PDA) culture medium at 25°C for one week. For strain activation, a sample of the material was cultured on Clavel agar (CLA) to facilitate the observation of macroconidia at a temperature of 28°C for 48 h.
Evaluation of the response of Passiflora edulis f. flavicarpa to F. oxysporum
Seeds of the selected accessions of three replicates were germinated according to the protocol proposed by Cardona et al. (2005). The seeds were first disinfected. Sterile organically fertilized soil in black bags of 1 kg was used for adequate development of the plants. The emerging seedlings were kept under greenhouse conditions with temperature between 20±1°C (measured with a thermo-hygrometer Traceable Brand, Fisher Scientific, Pittsburgh, PA, USA). Relative air humidity 77.5±2% and luminosity between 650 μmol m2 s-1 were measured with a luxmeter Apogee MQ-100 (Apogee Instrument, Logan, UT, USA); these were favorable conditions for the establishment of the P. edulis and the pathogen (Ramos et al., 2017).
Inoculations were made on 5-month-old plants with stem diameters of 10-12 mm using the average of 10 leaves. Tests were carried out by performing a stem incision on completely healthy plants. A deep cut was made at the base of the stem with a sterile blade and the inoculum was added per 5 ml per plant with a concentration of 3x106 macroco-nidia/ml (Ángel-García et al., 2018).
Mature leaves of the middle third stratum were evaluated for stomatal conductance measured by porometer (Decagon Devices, Pullman, WA, USA) (±10%), and chlorophyll content was measured with the Apogee chlorophyllometer MQ-100 (Apogee Instrument, Logan, UT, USA) (± 1%). The pre-inoculation symptoms at 4, 12, 20, and 28 d after inoculation (dpi) were monitored. Physiological values were taken at predawn (04:00 am) and at midday (12:00 pm) (Pérez & Melgarejo, 2014). Three plants for each accession were used as a negative control and were inoculated similarly with distilled water. The response was evaluated morphologically by identifying the degree of chlorosis in the leaves and by measuring the area with the ImageJ as follows: 0% without symptoms; 10% to 40% of the leaf area with yellowing; 60% to 80% of the leaf area with mild leaf and stem with necrosis; and 100% necrosis.
To confirm the presence of the pathogen and its association with the symptoms, a re-isolation was performed from the leaves and roots with a destructive method in PDA culture medium (Hernández et al., 2019) followed by a subsequent evaluation of color and shape of the colony and conidia to comply with Koch's postulates (Fig. S1).
Statistical analysis
Inoculations were carried out under a completely randomized design using three seedlings that were 5 months old for each accession as well as the control group. Phenotypic variables were evaluated using descriptive statistics, normality tests (with logarithmic transformation for variables without normal distribution), and analysis of variance to determine variations between the landraces and cultivars. To determine variables that explained the diversity among the varieties to a greater extent, the variance inflation factor (VIF) was determined; and a principal component analysis was performed using the RWizard package (Guisande et al., 2014). The results of the qualitative and quantitative descriptors were analyzed simultaneously using the Ward-MLM method to group the landraces and similar cultivars (Franco et al., 1998; Paiva et al., 2014). This procedure was carried out in the SAS v. 9.4 program (SAS Institute Inc, Cary, NC).
Results
Morphological and physiological descriptors of landraces and cultivars
A total of 46 cultivars and 17 local varieties were collected, and each was labeled as an accession. The samples were collected in the departments of Boyacá, Casanare, Cundinamarca, Meta, and Quindío at altitudes between 400 and 1,900 m a.s.l. Averages of the morphophysiological evaluations are presented in Table 1, indicating the mean, standard deviation, normality, and the variance inflation factors (VIF). The representative values are depicted in red. Considering the VIF, only 15% of the variables showed variability between cultivars and landraces (Tab. 2). Among the morphological descriptors, the following stand out for the cultivars (first value shown) and land-races (second value): °Brix (13.06±3.6 and 11.85±3.1), the percentage of germination (49.85±25.6 and 55±24.3%), the number of seeds per fruit (168.19±8.4 and 150.85±4.3), the leaf area (142.23±9.6 and 84.08±6.5 cm2), the length of the flower filament (1.0±0.1 and 0.88±0.1 cm) and of the anther (1.30±0.2 and 1.28±0.1 cm), and the length of the style (1.42±0.2 and 1.34±0.3 cm). Similarly, the physiological parameters that show variation between these groups (cultivars and landraces) are the evaluations carried out at midday for stomatal conductance (4.74±7.9 and 69.09±4.8 mmol m-2s-1) and chlorophyll content (481.07±30.4 and 497.31±25.8 μmol m-2).
According to the normality test (Shapiro test with a significance level of 95%), the descriptors of leaf area, anther length, and transverse stigma length did not have a normal distribution, according to the P-value of 0.05. For this reason, these variables were transformed for subsequent analyses.
TABLE 1 Quantitative descriptors evaluated in landraces and cultivars of P. edulis f. flavicarpa.
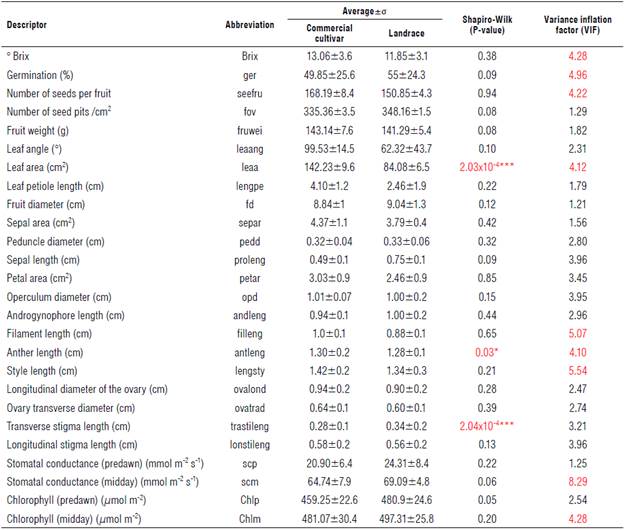
The values correspond to the average n=3. σ - standard deviation. Values in red indicate significant differences according to the VIF test.
Some qualitative descriptors also showed variations between the landraces and cultivars; thus, the landraces produced seeds that were rounder than they were oval (Fig. 1A). The proportion of these was similar between the cultivars as well the color of the mature fruits (Fig. 1B). The most frequent color of the landraces was a yellowish-orange with a percentage greater than 40%, and none displayed an orange color. Conversely, the cultivars only had 10% fruits showing a yellowish orange color and there were no fruits with a red-orange color.
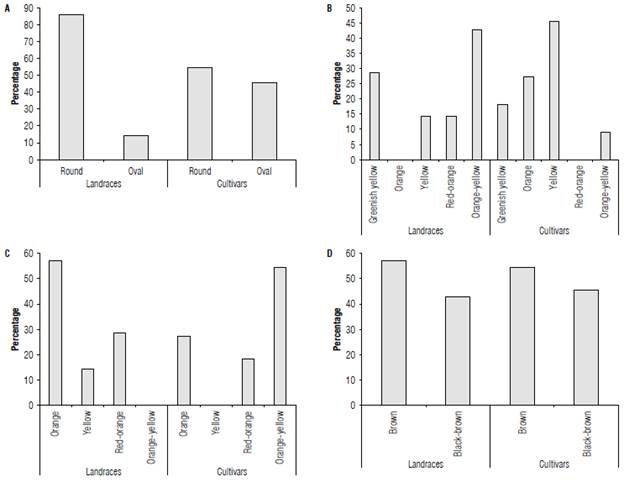
FIGURE 1 Qualitative fruit descriptors (%)for landraces and cultivars of Passiflora edulis f. flavicarpa. A) fruit shape, B) ripe fruit color, C) pulp color, and D) seed color. An average of 46 cultivars and 17 landraces are presented. n=3.
The color "orange" for the pulp of the landraces was dominant compared to the other colors (Fig. 1C). Orange-yellow was not represented for this group. In sharp contrast, the cultivars had the highest prevalence of orange-yellow color, while red-orange was not found. The color of the seeds (Fig. 1D) was very similar for cultivars and landraces: They exhibited light-brown and brown-black seeds.
In the analysis of principal components performed for the flower descriptors (Fig. 2), the principal component CP1 explains 42.12% of the variation in the data where the landraces (UPTC 1, 7, 19 and 20) are located with differential values for two of the cultivars (UPTC 3 and 10). Similarly, the vectors of style length (styleng), stigma length (lonstileng), and operculum diameter (opd) were more relevant for CP2, explaining 14.58% of the variation in the data. Here, the vectors of the variables regarding the length of the sepal length (proleng) and filament length (filleng) were more relevant.
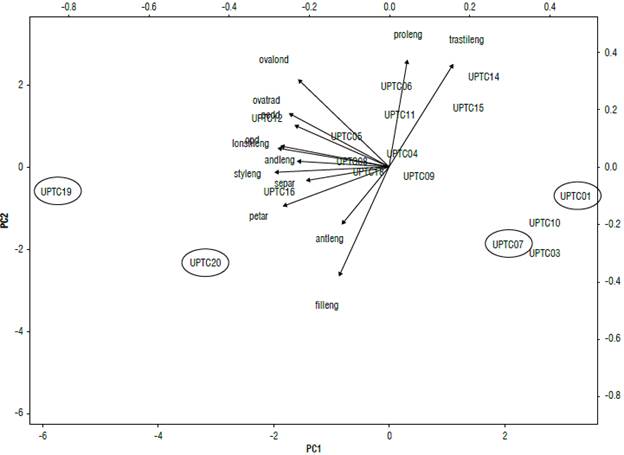
FIGURE 2 Principal component analyses (PCA) of the morphological characteristics of the flowers: sepal area (separ), floral peduncle diameter (pedd), sepal length (proleng), petal area (petar), operculum diameter (opd), androgynophore length (andleng), flower filament length (filleng), anther length (antleng), style length (styleng), ovary longitudinal diameter (ovalond), ovary transverse diameter (ovatrad), transverse stigma length (trastileng), and longitudinal stigma length (lonstileng). An average of 46 cultivars and 17 landraces of Passiflora edulis f. flavicarpa is shown. n=3. The accessions were named as UPTC.
Response of landraces and commercial cultivars to Fusarium oxysporum
According to the results of morphological variation, 18 accessions including 12 cultivars and 6 landraces were selected (landraces: UPTC 1,7,8,9,9,19 and cultivars: UPTC 2, 3,4, 5,6,10,11,12,14,15,16,20) and were inoculated with F. oxysporum. Prior to inoculation with F. oxysporum the plants had the chlorophyll content with average values of 574±130 μmol m-2 for the landraces and 596±156 μmol m-2 for the cultivars. Evaluations were carried out every 8 d for one month, and they indicated that the concentration of chlorophyll was reduced for 28 dpi to 481±125 μmol m-2 in the landraces and 458±108 μmol m-2 in the cultivars. Similar values for the landraces were seen in the control group, where the chlorophyll concentration fluctuated between 478.5±51.9 and 570.1±128.1 μmol m-2 (Fig. 3).
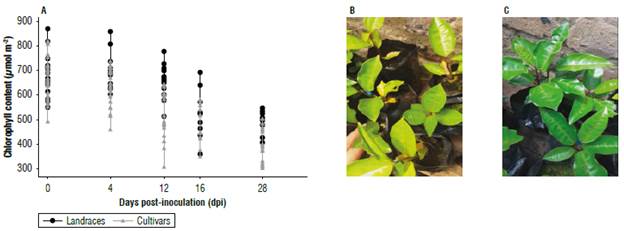
FIGURE 3 Relative chlorophyll contents (μmol m-2) in leaves of Passiflora edulis f. flavicarpa. A) evaluation from day 0 (prior to inoculation) up to 28 dpi with F. oxysporumot landraces (black circle) and cultivars (grey triangle). An average of 12 cultivars and 6 landraces is shown, each collection equal to n=3. Degree of chlorosis of cultivars (B) is compared to that of landraces (C) at 28 dpi.
The plant water status associated with the potential photosynthetic rate evaluated through stomatal conductance showed that the average values were higher in the landraces on each of the sampling days compared to the cultivars (Fig. 4). These values indicated that the midday stomatal conductance before inoculation totaled an average of 37.2 mmol m-2 s-1 in the landraces, with a daily range that fluctuated between 15 and 180 mmol m-2 s-1, and an average of 100.4±28 mmol m-2 s-1 in the cultivars, with a daily range that fluctuated between 25 and 230 mmol m-2 s-1. These averages held even without many variations during the 20 dpi, while on day 28 dpi, the average conductance in the landraces was reduced to 98.1±35 mmol m-2 s-1, while in the cultivars the average increased to 126±29 mmol m-2 s-1. The cultivar UPTC 14 was the one that had an atypical value of 251±29 mmol m-2 s-1. Comparatively, the control group showed a much lower conductance value than both groups, exhibiting values that ranged between 19.7±6.7 and 48.2±15.1 mmol m-2 s-1.
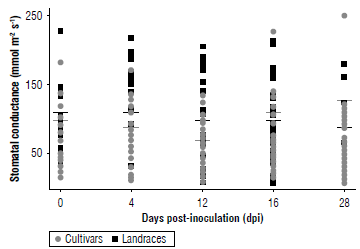
FIGURE 4 Stomatal conductance (gs) measured in landraces (black box) and cultivars (grey circle) of Passiflora edulis f. flavicarpa (mmol m-2 s-1) from day 0 (before inoculation) to 28 dpi with F. oxysporum. The black line shows the average of the landraces, while the gray line shows the average of the cultivars. An average of 12 cultivars and 6 landraces is presented, each collection represents n=3.
As a result of the above, we show that the ecophysiological parameters of chlorophyll content and stomatal conductance had variations between the landraces and cultivars in the predawn and midday samplings. Variations were also seen in relation to the symptomatologic scale after inoculation with F. oxysporum (Fig. 5). Chlorophyll values remained similar during predawn and midday, although landraces always had a higher chlorophyll content tan cultivars. The average predawn chlorophyll content for landraces and cultivars was 481±85 and 458.4±63.0μmol m-2 and at midday it was 497.3±125.0 and 481±143 μmol m-2. Likewise, the degree of chlorosis between landraces and cultivars had a percentage of 0.43±0.20% and 17.3±3.2% and reached values of 48% in the cultivars. Stomatal conductance significantly varied (P<0.05) between predawn and midday, with small variations between landraces and cultivars with the average values 26.0±2.9 and 21.7±5.7 mmol m-2 s-1 at predawn and 90.8±14.9 and 87.1±34.1 mmol m-2 s-1 at midday. Additionally, Pearson's correlation analysis showed a direct relationship between chlorosis and chlorophyll content at midday (r=0.81, P-value=0.01) and predawn (r=0.76, P-value=0.02) in landraces, and midday (r=0.76, P-value=0.02) and predawn (r=0.66, P-value=0.01) for the cultivars. In the same way, chlorosis was directly related with stomatal conductance at midday (r= 0.76, P-value=0.02) and predawn (r=0.69, P-value=0.01) for land-races and at midday (r= 0.73, P-value=0.01) and predawn (r=0.67, P-value=0.01) for cultivars.
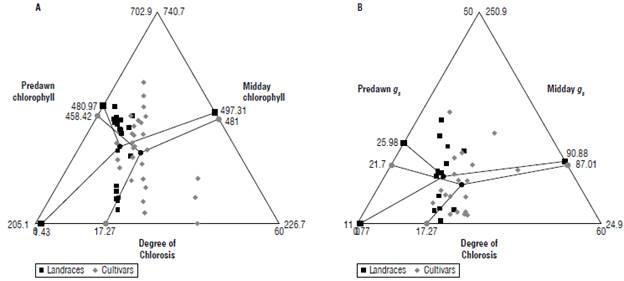
FIGURE 5 Correlation between ecophysiological parameters and the degree of chlorosis of Passiflora edulis f. flavicarpa at 28 dpi with F oxysporum. A) Triplot showing chlorophyll content at predawn=4:00 h (left) and midday =12:00 h (right) in relation to the degree of chlorosis (bottom), B) triplot showing stomatal conductance (gs) at predawn=4:00 h (left) and midday=12:00 h (right) in relationship to the degree of chlorosis (bottom). The landraces are shown with a black square and the cultivars are shown with a gray circle. An average of 12 cultivars and 6 landraces is shown, each accession comprises n=3.
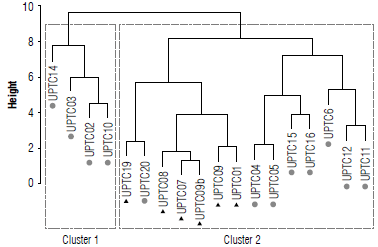
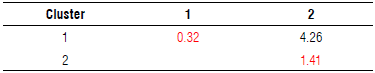
The landraces and cultivar accessions were separated according to morphological descriptors (qualitative and quantitative) and their response to F. oxysporum with physiological parameters. Two groups or clusters were defined according to Ward-MLM procedure (Tab. 3). The distances found indicated that there was greater diversity between the varieties of Cluster 2 (1.41) than between the varieties of Cluster 1 (0.32), while the variation between the two clusters was 4.26. This discrimination was confirmed with the Euclidean distance between the clusters that was 6.3 (not tabulated).
TABLE 3 Distance between the clusters formed by Ward-MLM and the intracluster distance on the diagonal in red. An average of 11 cultivars and 7 landraces of Passiflora edulis f. flavicarpa is shown, each collection consists of n=3.
Cluster 1 was made up of the cultivars UPTC 2, 3, 10, and xl4 (Fig. 6). All of the cultivars came from municipalities in Boyacá. While Cluster 2 contained all the landraces in one subgroup UPTC 19,20,8,7,9,9b, 1, the other cultivars contained cultivars in another subgroup, UPTC 4, 5, 15, 16,6, 12, and 11.
Discussion
The morpho-agronomic descriptors did not show high variations between landraces or cultivars, except for 23% of the descriptors (6 variables). Some characteristics of cultivars such as Brix degrees, number of seeds, and leaf area are highlighted that show higher averages due to selection processes of passionfruit populations (Jaramillo et al., 2009; Ocampo et al, 2013; Rodríguez Ambachew et al, 2020). Within the descriptors that did not show variation were the size and weight of the fruits. Under favorable conditions, the weight of these fruits range between 150 and 200 g (Dorado et al, 2013). The weights reported in our research were lower, with landraces weighing 141.3±5.4 g and cultivars weighing 143.1±7.6g. This may be because most of the landraces that were part of our study were located at higher altitudes in the department of Boyacá. They are new crops, meaning that good practices are still being established which include protocols for fertilizer application and other practices (Colombia Gobernación de Boyacá, 2017; MinTIC, 2020). In the landraces, low values are expected since mineral nutrients must be provided by mineralization of the soil's organic matter (Julca-Otiniano et al, 2006).
Other descriptors that distinguished these groups are related to the flower, such as the length of the anthers and the diameter of the stigma. Passionfruit is an allogamous plant that has structures in the flower to generally attract bumblebees that assist in pollination (Arias-Suárez et al, 2014). Thus, in the principal component analysis carried out with the floral morphological data (Fig. 2), it was possible to associate the characteristics that passionflowers use to facilitate fruit production. These include the dimensions of the stigma and the androgynophore that are both relevant (Bonilla et al, 2015) and whose vectors are located on the PCI. This explains most of the data. The diameter of the operculum and style length were also added (Rodriguez Ambachew et al, 2020; Ocampo et al, 2021). For PC2, the anther performs a different movement from the stigma, promoting attraction of pollinators (Bonilla et al, 2015). Also, the length of the filament and the length of the unifacial process of the sepal are important for pollination (Rodriguez Ambachew et al, 2020). For the ACP (Fig. 2), it was not possible to show a clear association of the land-race variations with respect to the cultivars; even so, the landraces UPTC 1,7,19, and 20 are located with high values on the PCI, indicating the importance of exploring these populations.
The characteristics associated with color, size, shape, and scent of the flowers make them efficient in pollination (Ramirez, 2006; Siqueira et al, 2009). Therefore, no matter how small the variations in these descriptors (according to our measurement systems and equipment) it is likely that pollinators have a different appreciation for these mechanisms. One expects that there is a wider range of pollinators in landraces than in cultivars (Ricketts et al, 2008). This is partly due to the use of agrochemicals that have drastically impacted pollinating insects to the point that, for many passionflower crops, manual pollination is necessary, increasing the costs of the crop due to the increase in labor (Bogdanski, 2008; Calle et al, 2010).
An evaluation of the responses of passion fruit accessions to F. oxysporum was carried out according to the Symptomatologie scale (Tab. 1). The landraces and cultivars behaved differently (Figs. 3B-C and 6A) since the landraces showed on average less than 1% chlorosis, indicating the absence of symptoms, while the cultivars showed 17% chlorosis with moderate yellowing. In general, these values were low and were like those obtained for another passionflower, where 2.5-month-old Passiflora maliformis plants at 28 dpi showed leaf decay and mild generalized chlorosis (Forero et al., 2016). This chlorosis can be caused by F. oxysporum advancing through the roots and plugging the vascular bundles that serve as transport of essential mineral nutrients for the plants. This influences on the content of many molecules, including chlorophyll (Fischer & Rezende, 2008). The content of chlorophyll was evaluated because it is a molecule that allows the determination of the physiological status of the plant. Chlorosis was not caused by a deficiency of mineral nutrients (such as magnesium) in the soil since a fertilized soil was used for this study. Chlorophyll is responsible for light absorption and through photosystems it allows photosynthesis (Lodish et al., 2016). An evaluation of this pigment identifies the level of stress that plants reach because of pathogens (Aguilar et al., 2012; Pérez & Melgarejo, 2014; Carmona et al., 2020). Our results showed a reduction in chlorophyll content from day 12 and up to 28 dpi (Fig. 3). This is associated with a reduction in the photo-synthetic rate so that its efficiency is impacted (Rodríguez & Cayón, 2008). The plant uses its protein structure to activate its defenses against pathogens, restricting other metabolic processes. The level of decrease in chlorophyll content was on average 300 μmol m-2 for landraces and cultivars (Fig. 3A) and was observed in relation to the degree of severity (Fig. 3B-C) that was measured by the external characteristics (Tab. 1). Chlorosis was evident for the cultivars, reaching a range of moderate chlorosis that differed from the landraces since these did not present external change. As no reference values were found for the chlorophyll content in this species when measured with a chlorophyll meter, we estimated that this reduction places the leaves in a range of moderate chlorosis. Additionally, similar studies where the degree of chlorophyll (extraction and quantification by spectrometry) also measured the severity of the disease caused by Fusarium revealed a mild generalized chlorosis at 28 dpi, after which the level of chlorosis increased (Ortiz & Hoyos, 2012; Forero et al., 2016).
Stomatal conductance is a parameter that indicates the condition associated with water deficit due to stomatal closure that can be caused by pathogens such as F. oxysporum (Fischer et al., 2009; Nankishore & Farrell, 2016; Carmona et al., 2020). This affects the normal transport of water and minerals through the xylem and causes variations in gas exchange that is consistent with the results observed for the chlorophyll content (Bishop & Cooper, 1983; Carmona et al., 2020; Aguilar et al., 2012). This is because stomatal closure also reduces CO2 entry and, therefore, carbon fixation, suggesting fewer active photosystems and reduced chlorophyll content (Aguilar et al., 2012). However, stomatal conductance values did not show significant variations between the landraces or cultivars with respect to this parameter (P=0.26) (Fig. 4). Research demonstrated that the decrease in the rate of net chlorophyll assimilation was associated with low values of stomatal conductance, limiting the gas exchange of the gulupa (Passiflora edulis f. edulis) plants once they were affected by F. oxysporum, with average initial values of 70 mmol m-2 s-1 before inoculation (Aguilar et al., 2012). At 28 dpi, the values were less than 10 mmol m-2 s-1 that contrasts with the results obtained in our study showing an average of around 100 mmol m-2 s-1, in general (Fig. 4).
As evaluated through measurements at predawn and midday for landraces and cultivars (Fig. 5B), stomatal conductance values varied throughout the daily cycle. Values close to 20 mmol m -2 s-1 were reported for the cultivars at predawn, and at midday they increased to a range of 50 to 90 mmol m-2 s-1. The landraces had higher values since they reached an average maximum of 40 mmol m-2 s-1 at predawn and a range of 78 to 110 mmol m-2 s-1 at midday. Studies carried out on gulupa indicate that midday stomatal conductance have higher values compared to the morning values, since this phenomenon is normal in C3-type plants (Sánchez et al., 2013; Pérez & Melgarejo, 2014).
Based on the simultaneous analysis of all the morphoag-ronomic and ecophysiological parameters, two clusters were organized. The first cluster was made up of cultivars (UPTC 02, 03, 10, and 14) from two municipalities in the department of Boyacá, where these crops were recently being introduced. The second cluster brought together the other cultivars (UPTC 04, 05, 06, 11, 12, 15, and 16) and all the landraces. This group was then separated into two subgroups, one comprising a landraces collection (UPTC19) originating from the department of Quindío together with a cultivar (UPTC20) from the department of Cundinamarca. The other subgroup was made up only of the landraces (UPTC 01, 07, 08, and 09) from Boyacá and Meta.
In addition, from the morphological variability it was evident there was a wide physiological response to the pathogen. These results confirmed the importance of continuing with the evaluation of landraces and wild populations that could provide greater genetic diversity to strengthen passion fruit cultivation against diseases such as those caused by phytopathogenic fungi such as F. oxysporum (Cerqueira-Silva et al., 2016). This is of greater importance in allogamous plants that need an increase in their variability to guarantee the sustainability of their cultivation and commercialization and to continue organizing seed banks in the areas where the species is naturally distributed and is currently affected by livestock farming (Hernández & García, 2006; Ocampo, 2013).
Conclusions
The morphological and ecophysiological characterization of yellow passion fruit populations against the effects of phytopathogenic organisms such as F. oxysporum allows an adequate analysis of the potential of landraces and cultivars. It is necessary to find a more efficient selection process, supported by the fact that some of the landraces have potential tolerance against this pathogen that is superior to that of the cultivars. This has led to an adaptation of the different populations and their associated biotic conditions. Therefore, it is necessary to expand evaluation studies of the cultivars and landraces to continue advancing research programs aimed at preserving this diversity in situ and ex situ in pro of food security.